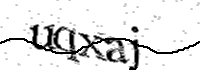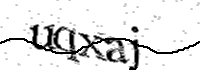Validation requestUser validation required to continue..
Please type the text you see in the image into the text box and submit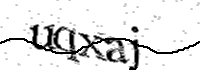
[ Refresh the page to generate a new image. ]
Note:
- If you get here while trying to submit a form, you may have to re-submit the form.
- Access to this domain may need the browser to have javascript and cookie support enabled.
Validation needed due to the detection of invalid input from this client IP address, error code : 421
Number of attempts left : 5