Functional Design Medication Process 9 version 3.0.0-beta.4 English version
![]() Klik hier voor de Nederlandse live-versie
Klik hier voor de Nederlandse live-versie
For an overview of relevant wiki pages for Medication Process see Landingspagina Medicatieproces
Inhoud
- 1 Introduction
- 2 Medication process
- 2.1 Process: medication verification
- 2.2 Process: prescribe
- 2.2.1 Current situation
- 2.2.2 Precondition
- 2.2.3 Trigger event
- 2.2.4 Process step: Evaluating a pharmaceutical treatment
- 2.2.5 Process step: Making a medication agreement
- 2.2.6 Process step: Making a variable dosing regimen
- 2.2.7 Process step: Creating a dispense request
- 2.2.8 Process step: Sending renal function value with prescription
- 2.2.9 Process step: Sending height and weight values with prescription
- 2.2.10 Process step: Send and/or make available
- 2.2.11 Postcondition
- 2.2.12 Information systems and transaction groups
- 2.2.13 Practical examples
- 2.3 Process: dispense
- 2.3.1 Current situation
- 2.3.2 Precondition
- 2.3.3 Trigger event
- 2.3.4 Process step: Providing pharmaceutical care
- 2.3.5 Process step: Contacting the prescriber
- 2.3.6 Process step: Creating an administration agreement
- 2.3.7 Process step: Supply
- 2.3.8 Process step: Send and/or make available
- 2.3.9 Postcondition
- 2.3.10 Information systems and transaction groups
- 2.3.11 Practical examples
- 2.4 Process: administer
- 2.5 Process: use
- 3 Domain-specific handling of the medication process
- 4 Description of practical examples
- 4.1 Practical examples, Prescribe
- 4.1.1 Short-term medication
- 4.1.2 Continuing medication
- 4.1.3 Hard endDateTime for PeriodOfUse
- 4.1.4 Medication as needed
- 4.1.5 Course of treatment as needed starting in future
- 4.1.6 Two dosages of the same medication at the same time
- 4.1.7 The same medicinal product with different strengths at the same time
- 4.1.8 Extra particularities in the medication agreement
- 4.1.9 New medication agreement, no dispense request
- 4.1.10 New dispense request under existing medication agreement
- 4.1.11 Dosage change (sufficient supply)
- 4.1.12 Prescription no longer needed after first dispense request
- 4.1.13 Discontinuing medication
- 4.1.14 Temporarily halting/resuming medication
- 4.1.15 Temporarily halting for an intervention
- 4.1.16 Paper prescription - no more allowed
- 4.1.17 Carrying out medication verification and evaluation of foreign or self-medication
- 4.1.18 Day treatment
- 4.1.19 Starting with medication before admission
- 4.1.20 Emergency admission
- 4.1.21 Interim discharge
- 4.1.22 Transfer to another institution
- 4.1.23 Do not dispense before
- 4.1.24 Discontinuation of medication by third parties
- 4.1.25 Two PRKs in a single pharmaceutical treatment
- 4.1.26 Creating a medication agreement after the fact
- 4.1.27 Single medication use
- 4.1.28 Provisional and final medication order
- 4.1.29 Inadvertently ‘outstanding’ medication or 'orphans'
- 4.1.30 Missing digital medication agreement at admission
- 4.1.31 Own articles (90 million numbers)
- 4.1.32 Free-text prescribing
- 4.1.33 Dosing with minimum interval
- 4.1.34 Dispense request with number of repetitions
- 4.1.35 Prescribing non-medicines
- 4.1.36 Send renal function value in the prescription
- 4.1.37 Cancelling a prescription that was sent earlier
- 4.1.38 Modification of someone else's medication agreement
- 4.1.39 Setting up a variable dosing regimen
- 4.1.40 Changing a variable dosing regimen during period of use
- 4.1.41 Stopping medication with a variable dosing regimen
- 4.1.42 Merging building blocks under one MBH
- 4.1.43 Changing someone else's MA by non-prescriber with delegated responsibility
- 4.2 Practical examples, Dispense
- 4.2.1 New medication agreement, medication dispense of the same product
- 4.2.2 New medication agreement, more precise product specification
- 4.2.3 Existing administration agreement is adequate
- 4.2.4 Proposal to prescriber for medication agreement
- 4.2.5 Request and dispense
- 4.2.6 Patient requests repeat prescription via physician (reactive repeat)
- 4.2.7 Patient requests repeat prescription via pharmacist
- 4.2.8 Proactive repeat prescription by pharmacist
- 4.2.9 Dispense based on an existing dispense request
- 4.2.10 Splitting a prescription
- 4.2.11 Starting and continuing a GDS
- 4.2.12 Pharmacist changes commercial product
- 4.2.13 Adding medication to a GDS
- 4.2.14 Discontinuing medication in a GDS
- 4.2.15 GDS supplier supplies other commercial product
- 4.2.16 Parallel administration agreements with GDS and non-GDS dispense
- 4.2.17 Parrallel administration agreements of which one is changed
- 4.2.18 Handling a stop-medication agreement
- 4.2.19 Dispense with someone else’s administration agreement
- 4.2.20 Modification of someone else’s administration agreement
- 4.2.21 Deviation from prescribed quantity in DispenseRequest
- 4.3 Practical examples, Administer
- 4.3.1 Creating an administration list
- 4.3.2 Exact administration times required
- 4.3.3 Missing (guide) administration times
- 4.3.4 Non-GDS medication as needed
- 4.3.5 Medication supply by multiple pharmacies
- 4.3.6 Change in GDS from the next supply or immediately
- 4.3.7 Increasing dosage of GDS in new MBH
- 4.3.8 Decreasing dosage of GDS in new MBH
- 4.3.9 Change processed by the pharmacist
- 4.3.10 Change not processed by the pharmacist
- 4.3.11 Extra instructions for administering, explanation in MA, TA and/or WDS
- 4.3.12 Medication administration deviates from administration list
- 4.3.13 Medication administration without medication agreement and administration agreement
- 4.3.14 Medication administration of self-medication
- 4.3.15 Correction of an administration
- 4.3.16 Medication not administered
- 4.3.17 Medication administration on hold
- 4.3.18 Medication administration by a prescriber
- 4.3.19 Multiple administration organisations
- 4.3.20 Feedback to patient through a medication adherence app
- 4.3.21 Registration (stop-)MA retroactively with interim MTD
- 4.3.22 Registration of separate MTDs per InjectionPatchSite
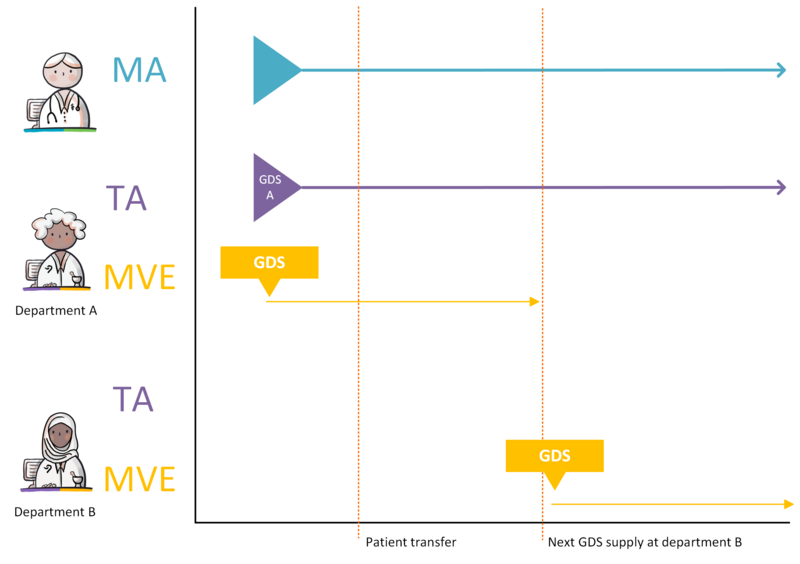
- 4.3.23 Patient transfer to a different department with the same GDS supply date
- 4.3.24 Patient transfer to a different department with an earlier GDS supply date
- 4.3.25 Patient transfer to a different department with a later GDS supply date
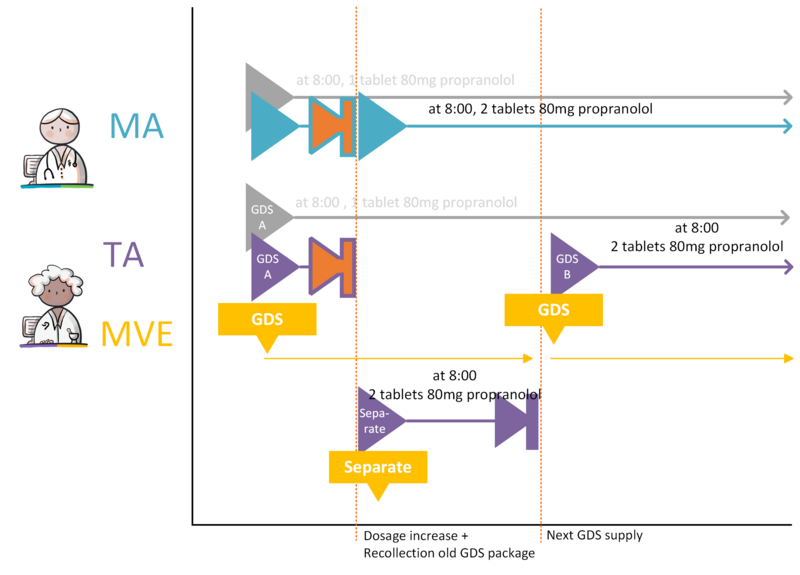
- 4.3.26 GDS medication dosage increase, no change in administration times (option A)
- 4.3.27 GDS medication dosage increase, no change in administration times (option B)
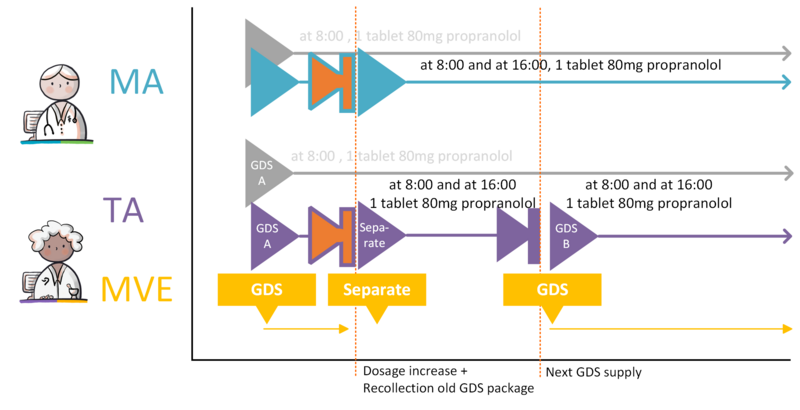
- 4.3.28 GDS medication dosage increase, change in administration times
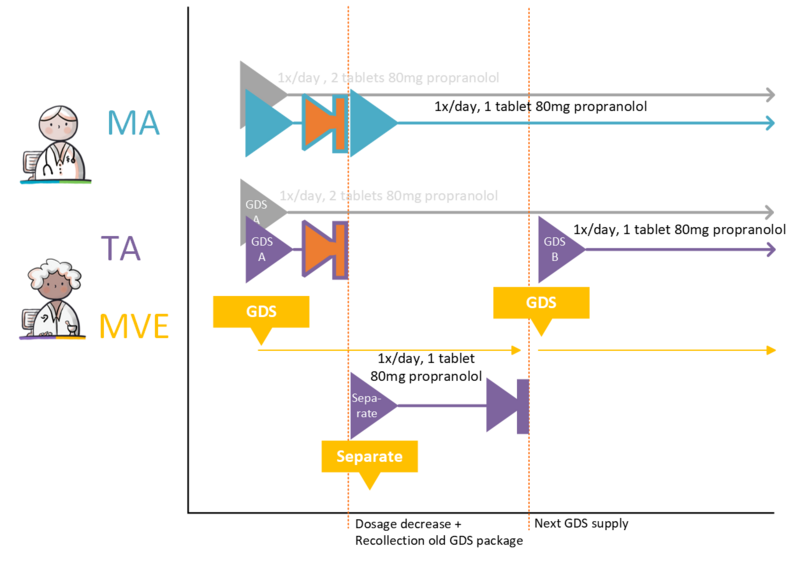
- 4.3.29 GDS medication dosage decrease
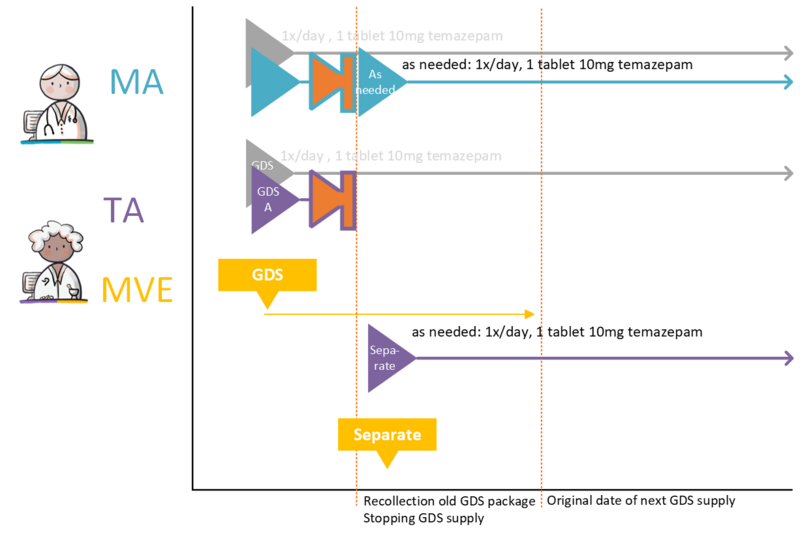
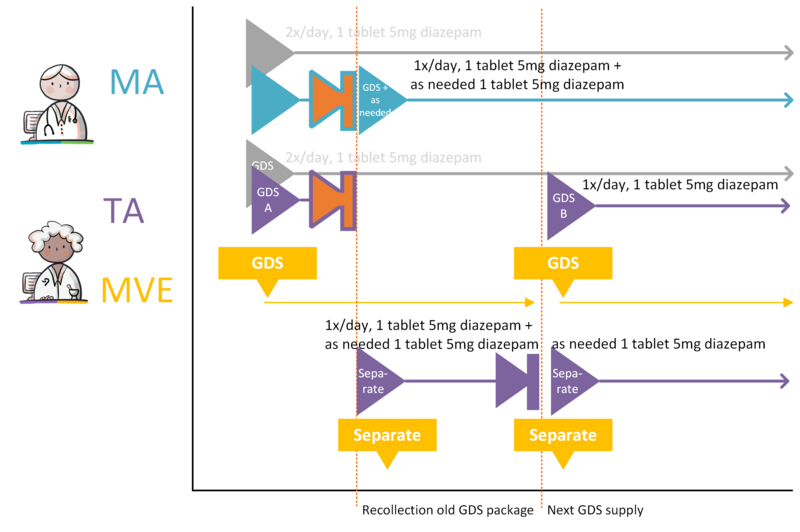
- 4.3.30 Changing GDS medication to ‘as needed’ medication
- 4.3.31 Splitting GDS medication into GDS medication and ‘as needed’ medication
- 4.4 Practical examples, Use
- 4.4.1 Self-care product
- 4.4.2 Medication from abroad
- 4.4.3 Modification on the patient’s initiative
- 4.4.4 Discontinuation of medication on the patient’s initiative
- 4.4.5 No more supply
- 4.4.6 Registrations of abnormal medication use by patient due to adverse drug reactions
- 4.4.7 Register medication use based on supply
- 4.1 Practical examples, Prescribe
- 5 Medication overview and inference rules
- 5.1 Introduction
- 5.2 Functional specification
- 5.3 Building block instantiations
- 5.4 Verification per MBH
- 5.5 Process of the medication overview exchange
- 5.6 Inference rules
- 5.7 Data that need not be shown
- 6 Administration list and inference rules
- 7 Information systems and transactions
- 8 Functionality
- 8.1 Filtering medication from 2nd/3rd line (all information systems)
- 8.2 Making medication data available (all information systems)
- 8.3 Notification date or dispense date (pharmacist information system)
- 8.4 Updating after system malfunction
- 8.5 Construction for ‘once every 36 hours’ interval
- 8.6 EVS / HIS processing Regulation processing
- 8.7 Pages with additional information relevant to the Kickstart
- 8.8 Medication use: UseIndicator, AsAgreedIndicator, MedicationUseStopType, PeriodOfUse and DosingInstructions
- 8.9 Implementation of medication distribution system (GDS) data elements
- 8.9.1 Background
- 8.9.2 Wishes of care providers
- 8.9.3 Data elements Medication process
- 8.9.4 Application of data elements to support desired functionality for GDS patients
- 8.9.4.1 Recording that someone uses GDS
- 8.9.4.2 Recording why someone uses GDS
- 8.9.4.3 Record for medication that this must be in the GDS
- 8.9.4.4 Establishing / exchanging the duration of a medication roll
- 8.9.4.5 Establishing / exchanging whether a change in the medication should be effective immediately or not
- 9 Reflections
- 10 Appendix References
- 11 Attachment: Document history
- 12 Appendix: Figures and tables
1 Introduction
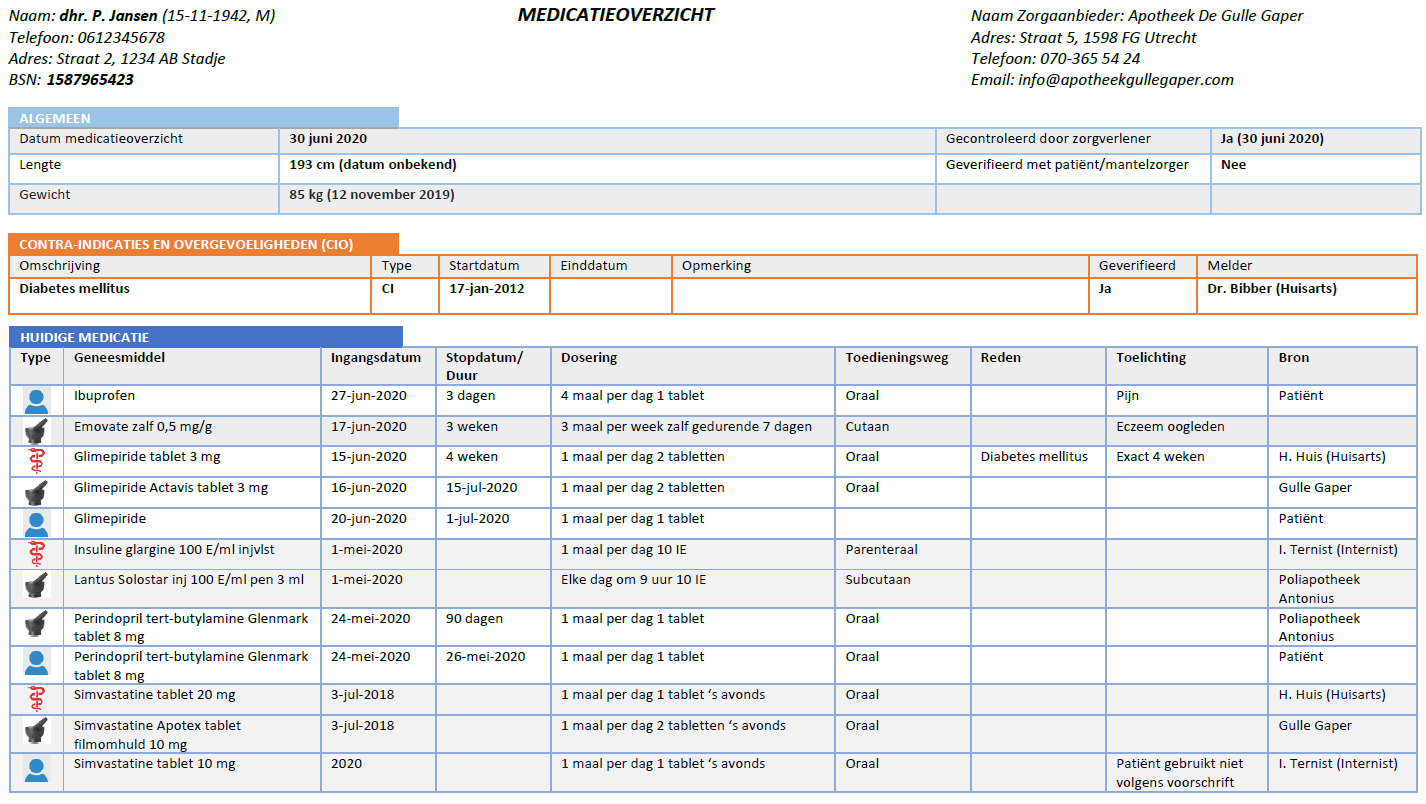
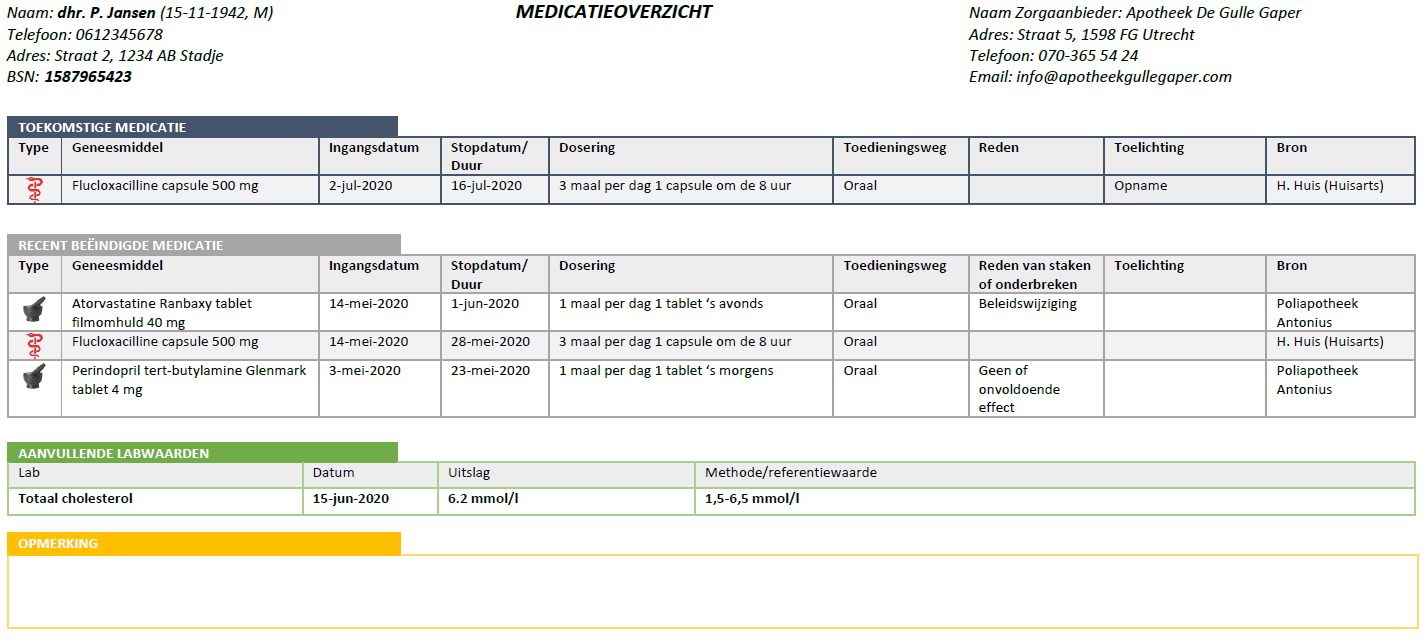
This document is the functional design for the Medication Process Information Standard (in Dutch: 'Informatiestandaard Medicatieproces'). It provides a general description as well as a description of specific practical situations. The recording and exchange of information is described for specific situations using actors (people, information systems) and transactions (which information is exchanged when).
Target groups for this document:
- Health professionals
- Information analysts and architects
- Software suppliers
1.1 Scope and vision
This document has been produced within the Medication process program. The Medication process program aims first to take away existing obstacles in the medication process, while taking into account current legislation and the possibility of obtaining tangible results in the foreseeable future.
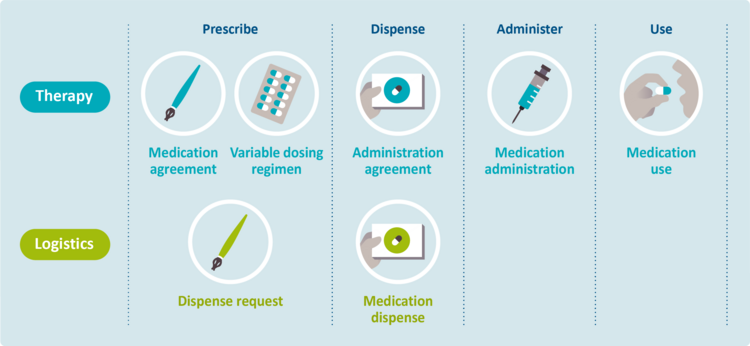
One of the main obstacles concerns the lack of insight in the actual medication use of patients. This is partly due to the fact that therapeutic and logistical information are often mixed, which results in the medication history becoming unclear. The following distinction between therapy and logistics exists:
- Therapy covers the medical side. This includes medication (and treatment) agreements, as well as the corresponding support and implementation. Therapeutic intention, (actual) medication use, self-medication and pharmacotherapy are also covered by the term ‘therapy’ as it is defined in this document.
- Logistics covers the physical flow of medicinal products, including requests, planning and dispense. This also includes the medication supply and consumption of this supply.
The program has taken into account current legislation and feasibility within the foreseeable future. The vision goes beyond the scope of the Medication process program and lays the foundations for a situation where a dispense request is no longer required. The ultimate objective is for prescribers to only have to concern themselves with the therapeutic side (which medicinal product, which strength, which dosage, when to start, etc.). It will no longer be necessary to create a dispense request. Instead, the prescriber will make medication agreements directly with the patient. Based on these medication agreements, the pharmacist will take care of the logistical process, eliminating the need for a dispense request altogether. Because of legislation this is not (yet) possible. The Medication process program does however take the first necessary step in the right direction.
1.2 Reading guide
The following paragraph introduces the main building blocks and the terminology used in this document. Detailed descriptions of the various processes (prescribe, dispense, administer, medication use) are given in Chapter 2. The purpose of the descriptions is to clarify how healthcare processes function in an ideal situation; which process steps are needed; which actors are participating; which information applies; and which moments of exchange exist. The process descriptions follow a fixed format:
- Current situation
This paragraph describes the relevant differences between the current situation and the desired situation ('soll') in accordance with this information standard. Any obstacles will be described here. - Process description with the paragraphs:
- Precondition
The conditions that must be met before the process is started. - Trigger Event
The event that starts the process. - One or more process steps
Description of part of the process. - Postcondition
The conditions that are met after the process steps have been carried out. - Practical examples
List of practical examples associated with a specific subprocess. These are detailed in Chapter 4. - Information systems and transaction groups,
This paragraph describes the information systems, system roles, transactions and transaction groups related to the process steps. All information concerning information systems and transaction groups is also included in Chapter 7.
- Precondition
Chapter 3 describes a number of domain-specific interpretations of the medication process, for instance those of the thrombosis service and those related to service observation services in an ambulatory situation. Chapter 4 describes several practical examples in more detail. These examples are, in a large number of cases, derived from general medical practice but are illustrative of similar situations in a different setting. The practical examples are classified according to subprocess, as indicated in Chapter 2.
Chapter 5 describes how a medication profile can be constructed from the different building blocks. Chapter 7 includes an overview of all information systems, system roles, transactions and transaction groups. Guidelines for the functionality of the various information systems have been detailed in Chapter 8.
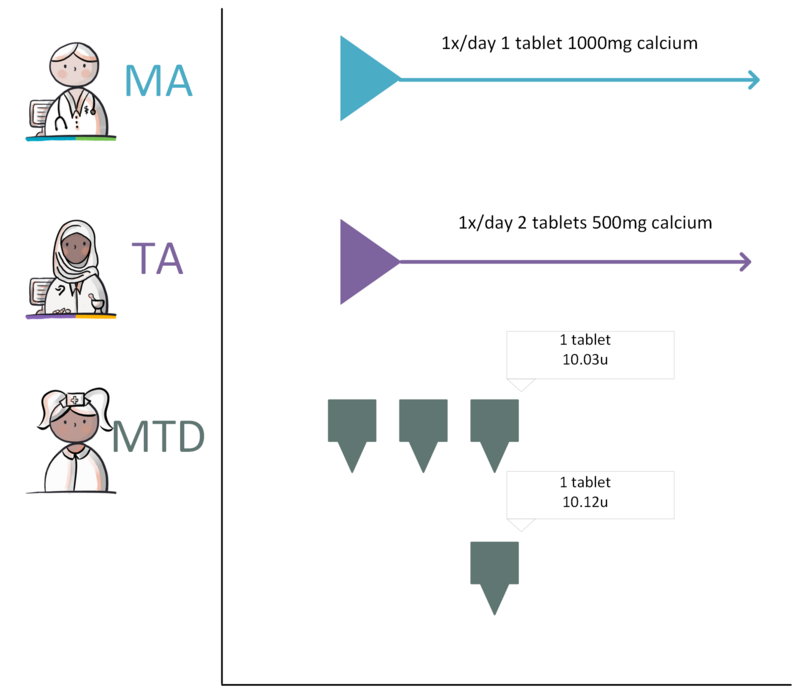
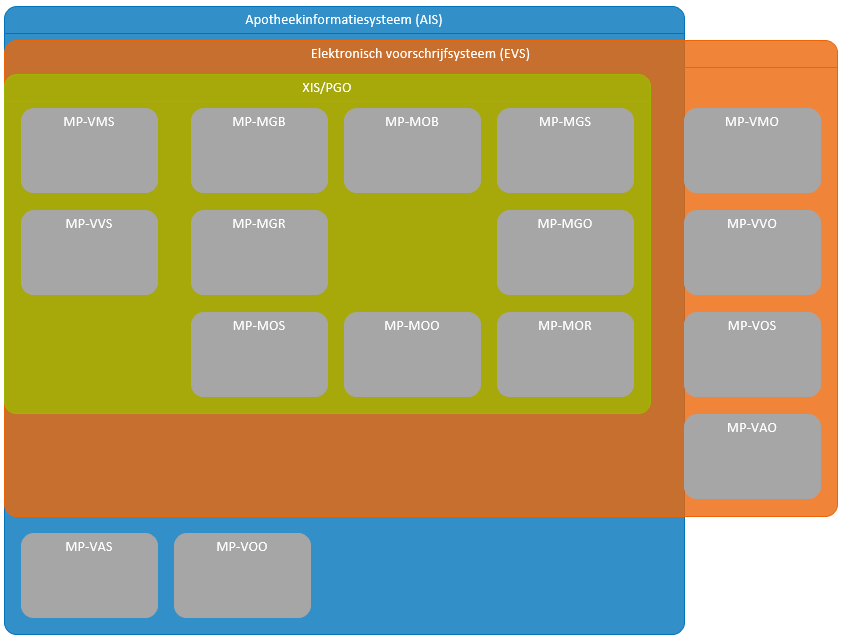
In this document, Dutch abbreviations are used for the medication building blocks (see Table 1 below).
1.3 Introduction of relevant terms
1.3.1 Therapeutic and logistical building blocks
Chapter 2 provides a description of the process and the data elements associated with it. Related data elements are grouped together in a Clinical Information Model (CIM) or Clinical Building Block (CBB) (in Dutch: 'zorginformatiebouwsteen' - zib). The dataset details the data elements these zibs consist of; data elements may have been added to the zibs in keeping with the clinical context and care process. The data set includes the complete set of definitions of the data elements of the building blocks. The building blocks together with their data elements can be used in various scenarios for arranging/modelling healthcare applications or for defining interfaces for data exchange.
The different building blocks are shown in the figure below. They have been ordered according to process and subprocesses, and according to therapy versus logistics.
The four additional concepts ‘Proposal medication agreement’ (therapeutic), 'Reply proposal medication agreement' (therapeutic), ‘Proposal dispense request’ (logistics) and ‘Reply proposal dispense request’ (logistics) are also described.
| Building blocks in Dutch | Abbr. in Dutch | Building blocks in English | Description |
|---|---|---|---|
| Medicatieafspraak | MA | Medication agreement | 'Medicatieafspraak / Medication agreement/ is the prescriber’s proposal for medication use with which the patient agrees. An agreement to discontinue medication is also an MA[1]. |
| Wisselend doseerschema | WDS | Variable dosing regimen | 'Wisselend doseerschema / Variable dosing regimen' contains the dosing instruction as composed by an (external) prescriber. In the WDS the element 'instructions for use' from the MA is further specified. The WDS can be modified without changing the MA. |
| Verstrekkingsverzoek | VV | Dispense request | 'Verstrekkingsverzoek / Dispense request' is the request from a prescriber to a pharmacist to supply the patient with one or more medicinal products in support of the current MA(s)[2]. |
| Toedieningsafspraak | TA | Administration agreement | 'Toedieningsafspraak / Administration agreement' contains the instructions for medication use (or administration) from the pharmacist to the patient (or his representative or administrator), adding to the MA[3]. |
| Medicatieverstrekking | MVE | Medication dispense | 'Medicatieverstrekking / Medication dispense' is the provision of a supply of medicinal product to the patient or his administrator or representative. |
| Medicatietoediening | MTD | Medication administration | 'Medicatietoediening / Medication administration' is the registration of individual administrations of the medicinal product to the patient by the person who administers them (such as a nurse or the patient himself) in relation to the agreements made. |
| Medicatiegebruik | MGB | Medication use | 'Medicatiegebruik / Medication use' is a statement about historical, current or intended use of a medicinal product[4]. |
| Voorstel medicatieafspraak | VMA | Proposal medication agreement | 'Voorstel medicatieafspraak / Proposal medication agreement' is a recommendation or request from the pharmacist, prescriber or the administrator to the prescriber of the MA about the medication agreed upon. The recommendation request may include evaluating, discontinuing, starting or modifying medication. |
| Antwoord voorstel medicatieafspraak | AVMA | Reply proposal medication agreement | 'Antwoord voorstel medicatieafspraak / Reply proposal medication agreement' is a reply from the prescriber to the VMA. |
| Voorstel verstrekkingsverzoek | VVV | Proposal dispense request | 'Voorstel verstrekkingsverzoek / Proposal dispense request' is a proposal from the pharmacist to the prescriber to approve one or more MVE(s) in support of the current MA(s). This is comparable with the current situation of submitting the authorisation form or combined prescription or submitting a repeat prescription for signing. The patient may also submit a VVV to the prescriber. |
| Antwoord voorstel verstrekkingsverzoek | AVVV | Reply proposal dispense request | 'Antwoord voorstel verstrekkingsverzoek / Reply proposal dispense request' is a reply from the prescriber to the VVV. |
Table 1 Building blocks – description
1.3.2 Medication overview
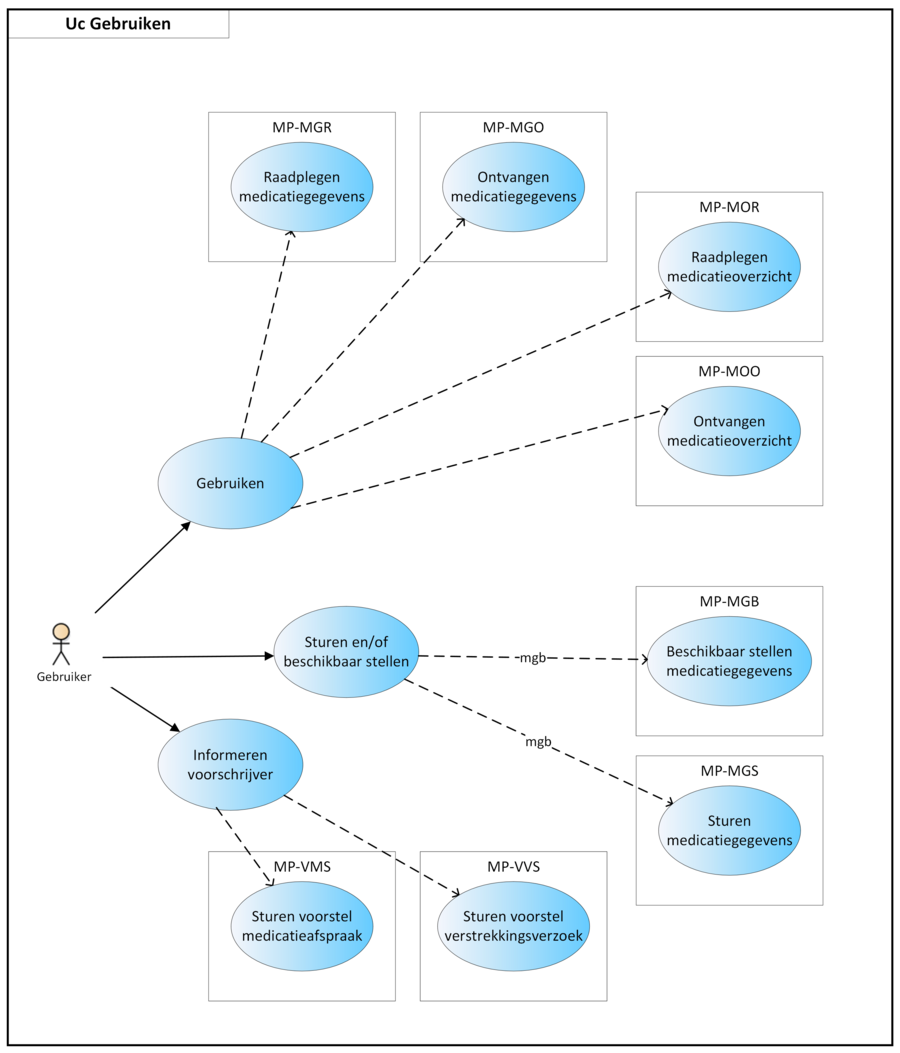
See Chapter 5 for more information about these overviews, the applicable building blocks and how a medication profile/current overview can be compiled.
1.3.3 'Medicamenteuze behandeling / Pharmaceutical treatment' (MBH)
The different medication building blocks represent steps in the medication process, from prescribing a medicinal product (MA and/or VV), followed by dispensing it (TA and/or MVE) up to and including administering and using the medicinal product. The model is designed in such a way that therapeutic building blocks and logistical building blocks are separated from each other.
Scope
In order to be able to illustrate the interdependence of the medication building blocks, the concept of ‘pharmaceutical treatment’ (in Dutch: 'medicamenteuze behandeling' - MBH) is introduced.
- 'Pharmaceutical treatment' is a technical concept in the information standard. Its purpose is:
- To unambiguously identify the set of interdependent medication building blocks, and
- To apply rules to it to unambiguously determine the present situation.
- To unambiguously identify the set of interdependent medication building blocks, and
The functional application of the concept of MBH is as follows:
- Medication (or MBH) is started by creating a first MA as part of a new MBH.
- Medication (or MBH) is discontinued by creating a new MA within the same MBH (stop-MA).
- Medication (or MBH) is modified by:
- Discontinuing the existing MA and
- Creating a new changed MA as part of the same MBH. The starting date (hereafter: startDateTime according to naming convention in the dataset) of this new MA may also be in the future.
Prescribing a new medicinal product always results in a new MA. An MA is always related to a single MBH. For the time being, the PRK level (Prescription Code from the G-standard) of the medicinal product determines whether the MA belongs to a new or an existing MBH. A detailed description can be found in paragraph 2.2.5.
Exceptions:
- Products without PRK (a non-medicine such as crutches or bandages). In this case the HPK level (Trade Product Code from the G-standard) determines if the MA will lead to a new MBH.
- Medication without PRK (magistrals often consist of several substances that are not covered by the same PRK, these substances are included separately as ingredients in the MA). Every magistral or modification thereof falls under an MBH.
- Own articles without PRK (articles listed in the internal information system under 90 million numbers stored, such as half tablets, commonly used magistrals). Any item or modification thereof falls under an MBH.
- When prescribing in free-text (meaning a prescription without a code from the G-Standard) every change of the product may lead to a new MBH.
- Intravenous (IV) therapy (to be worked out).
Examples
Five examples illustrate the scope of an MBH:
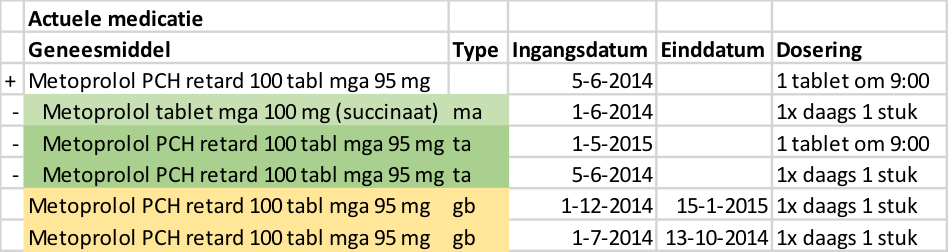
- Diazepam, 5 mg, 1 tablet 4x daily is changed to diazepam, 5 mg, 1 tablet 3x daily. The PRK level of both products is the same, they are both part of the same MBH.
- Paroxetine tablet, 10 mg, 1 tablet 1x daily, is changed to paroxetine tablet, 20 mg, 1 tablet 1x daily. This is a modification of an MA with two different medicinal products at the PRK level. This change requires that the first MBH is discontinued and a new MBH is started.
- A gastroprotective drug has been agreed upon in a treatment with prednisone: prednisone and gastroprotective drugs are two different medicinal products which are used parallel to each other, and their use can be modified and discontinued independently of each other. This means they are not part of the same MBH.
- Switching from a beta blocker to an ACE inhibitor means a new PRK and this is achieved by discontinuing the MBH of the beta blocker and starting a new MBH for the ACE inhibitor.
- When there is no PRK and the composition of the medicinal products in the MA changes (any change in the ingredients), the existing MBH is discontinued and a new MBH is started. This applies, for example, to extemporaneous preparations, drips and proprietary products.
Creation of an MBH
A schematic overview of how an MBH is set up can be seen in the figure below. When a new building block (MA, TA, VV, MVE, MTD or MGB) is created, a check is first performed to determine whether this is a new medication or if there already is a current building block with the same product. This applies to all building blocks, both own building blocks and third parties’. In most information systems, the user of the information system will indicate that he wants to change one of the existing medication building blocks or wants to introduce new medication. In that case, it is easy to find out whether there already is an MBH that includes the building block.
- If there is no existing building block for this medication, a new building block with a new MBH is created.
- If there is an existing building block with an MBH, the user of the information system is asked whether the new building block and the existing building block belong to the same treatment. When this is the case, the same MBH will be used. When the building blocks do not belong to the same treatment, a new MBH will be created.
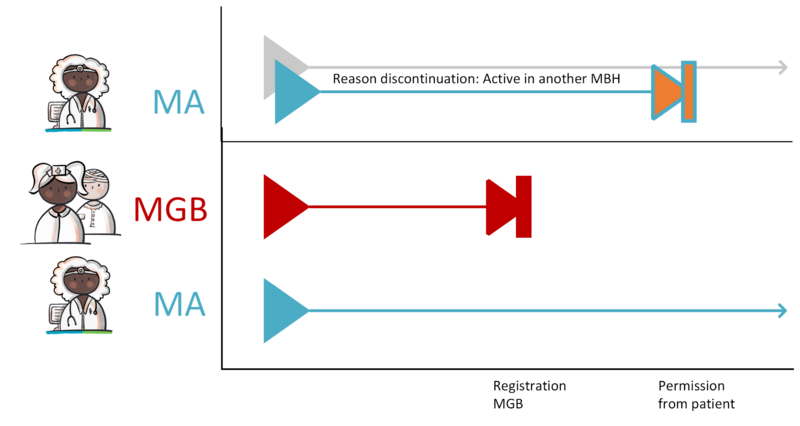
Situations can occur where an MBH is created when it shouldn't have been. For example when a patient has not given permission for sharing their medical information. The way of working for merging MBHs is described in the implementation guide for migration and hybrid situations. A practical example can be found in paragraph 4.1.41.
Parallel occurrence of medication agreements
Within an MBH, several MAs may be active simultaneously. These are all MAs that are valid ('current') at this time or that will become valid in the future. In principle, only one MA is valid at any time in an MBH. However, there are a number of situations where parallel MAs are conceivable:
- The same medicinal product, but a different strength, where the total strength should essentially be prescribed in one agreement.
- Related (different) medicinal products that are given together, but that should be considered as a whole when evaluating treatment.
- Technical omissions in information systems. For example, in the case of complicated dosing schedules or combination drips.
Situation 1 In this situation, it was decided to combine one or more products in the same MA by entering the products as ingredients. This is comparable to extemporaneous preparations. However, the instructions for use for all these products must be identical.[5]
Situation 2 is solved in different ways in different information systems, each with their own grouping mechanisms. It concerns the correlation between different medicamenteuze behandelingen. The information standard does not provide a universal grouping mechanism.
Situation 3 is the only situation in which parallel MAs are permitted under one MBH. Complex phasing-in and phasing-out schedules and combination drips may be included in one MA, but not all information systems support this. For those information systems, it is permitted to create parallel MAs within a single MBH.
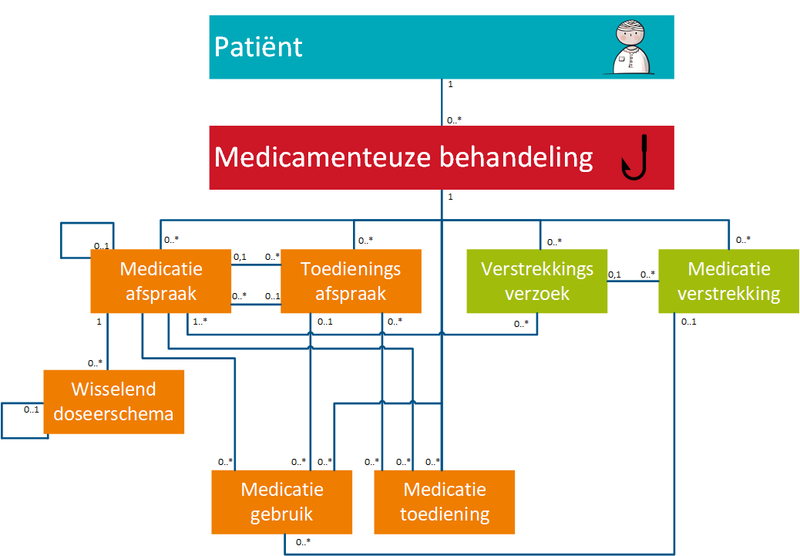
1.3.4 Correlation between building blocks and MBH
The figure below shows the correlations between building blocks and the MBH. The relations between building blocks and the MBH as well as the relations between the building blocks themselves are described as follows:
- Building blocks belong to a single MBH. An MBH usually includes at least one MA and may include zero or more of the following building blocks VV, WDS, TA, MVE, MGB and MTD. Unless, for example, self-medication has been recorded with MGB, a drug treatment can exist without an MA, but with MGB or TA. An MBH will never cease to exist, but it may no longer be effective when there are no current building blocks linked to it. VMA, AVMA, VVV and AVVV are not yet part of an MBH as these are still proposals that may or may not lead to a final MA or VV linked to an MBH. A VMA may lead to zero (if the recommendation is not followed), one or more MAs and a VVV may lead to zero (if the proposal is not honoured), one or more VVs.
- An MBH may also only have a stop-MA in addition to, for example, an MGB building block. For example, in the event that a health professional asks a patient to stop using free available medicine (self-medication or over-the-counter (OTC) medication). The health professional records the use of the self-medication in an MGB building block and discontinues the use by creating a stop-MA belonging to the same MBH.
- An MA may refer to the previous MA or a TA or MGB on which it is based. This may also be an MA, TA or MGB that belongs to another MBH. It is possible that no digital MA is available, this MA must then be created. This MA may refer to the TA or MGB. A pharmacist is never the source of an MA but he may have a copy.
- In principle, only one MA is valid at any one time in an MBH. Only when there are technical omissions in information systems, for example in case of complicated dosing schedules or combination drips, parallel MAs are allowed (see also the previous paragraph).
- An MA is supported by zero (if there is still enough supply or if no medication supply is needed), one or more (when there is, for example, continuous medication) VVs.
- A VV is based on the current MA and any existing corresponding TA in an MBH. There may be several.
- A VV refers to one or more MAs (for example, in the case of an interim dosage increase, a VV can be made that replenishes the supply for the existing MA and also starts the supply for the future MA).
- A VV may result in zero (for example when the patient does not pick up the medication) to several MVEs.
- An MA may result in zero, one or multiple WDSs.
- Multiple (possibly parallel) TAs may be based on the same MA (for example, when a pharmacist switches to a different commercial product or when the medicinal product is supplied as two or more medicinal products with different strengths, with the total strength remaining the same).
- An MA does not always have to lead to a TA, for example when no VV is required with a short use MA, when the patient still has sufficient stock.
- A TA is supported by zero (when there is enough supply), one or more MVEs.
- An MVE is based on an MA (and TA) and, in an ambulatory situation, on a VV. The exceptions are over-the-counter (OTC/self-medication) sales for the purpose of self-medication: these have no MAs and no VVs. Self-medication provided by the pharmacist may be recorded by that pharmacist as a TA with MVE, or as MGB by a random health professional or by the patient himself.
- An MVE may support multiple TAs.
- An MA or a TA may be followed by a new MA or TA. This may be the case when existing medication is changed (modification of MA and/or TA) or when MGB is discontinued (stop-MA/TA).
1.3.5 Send and/or make available
Throughout the medication process, information is being generated and used, including medication data. There are two working methods for the digital exchange of these data in the care chain, make available/consult and send/receive. Make available means that data in one's own information system are made available for consultation by other parties involved in the chain. Another possibility is to send data to other parties involved. These other parties receive the data automatically.
The data are sent to health care providers, not to specific health professionals. If in this FO mention is made of sending data to “the pharmacist/general practitioner/pulmonologist etc.” this concerns the corresponding health care provider, not that individual health professional.
Not all data are always or to everyone made available:
- Proposals and their answers are only sent between the person making the proposal and the recipient of the proposal, often the prescriber.
- Dispense requests are made available for patient consultation only.
- Height, Weight and Laboratory results can be sent with the medication prescription. Making available/consultation of these building blocks is not in scope of MP9.
The transfer of medication data as described in this document complies with laws and regulations. The Guideline Transfer of Medication data in the chain of care describes, among other things, the following:
- Explicit patient consent is required for making medication data available electronically by healthcare providers to other healthcare providers.
- No consent is required for non-electronic provision of medication data to healthcare providers directly involved in the execution of the treatment agreement.
- The sending of medication data to individuals directly involved in the execution of the treatment agreement (or their deputy) is allowed under the WGBO. This applies to individuals inside and outside the healthcare provider's organization.
Chapter 2 elaborates in which situations data should only be made available, or sent and made available. This is summarized per process in tables in the Compilation file (Verzamelbestand per proces).
1.4 Legend/Explanation
A manual for this Nictiz wiki documentation can be found at:
http://informatiestandaarden.nictiz.nl/wiki/Handleiding_Wiki_documentatie
It also includes a legend for the various figures that appear in this document.
2 Medication process
This chapter describes the medication process in relation to the building blocks for first-line, second-line and third-line health care. The process is fundamentally the same in each case. The main difference is which pharmacy supplies the medication: a community pharmacy (including an outpatient pharmacy) or a hospital pharmacy. Another difference is that in an ambulatory setting a VV is required for the supply of medicinal products. This is not required in a hospital setting: the (hospital) pharmacist ensures that the medicinal products are available as long as the MA continues.
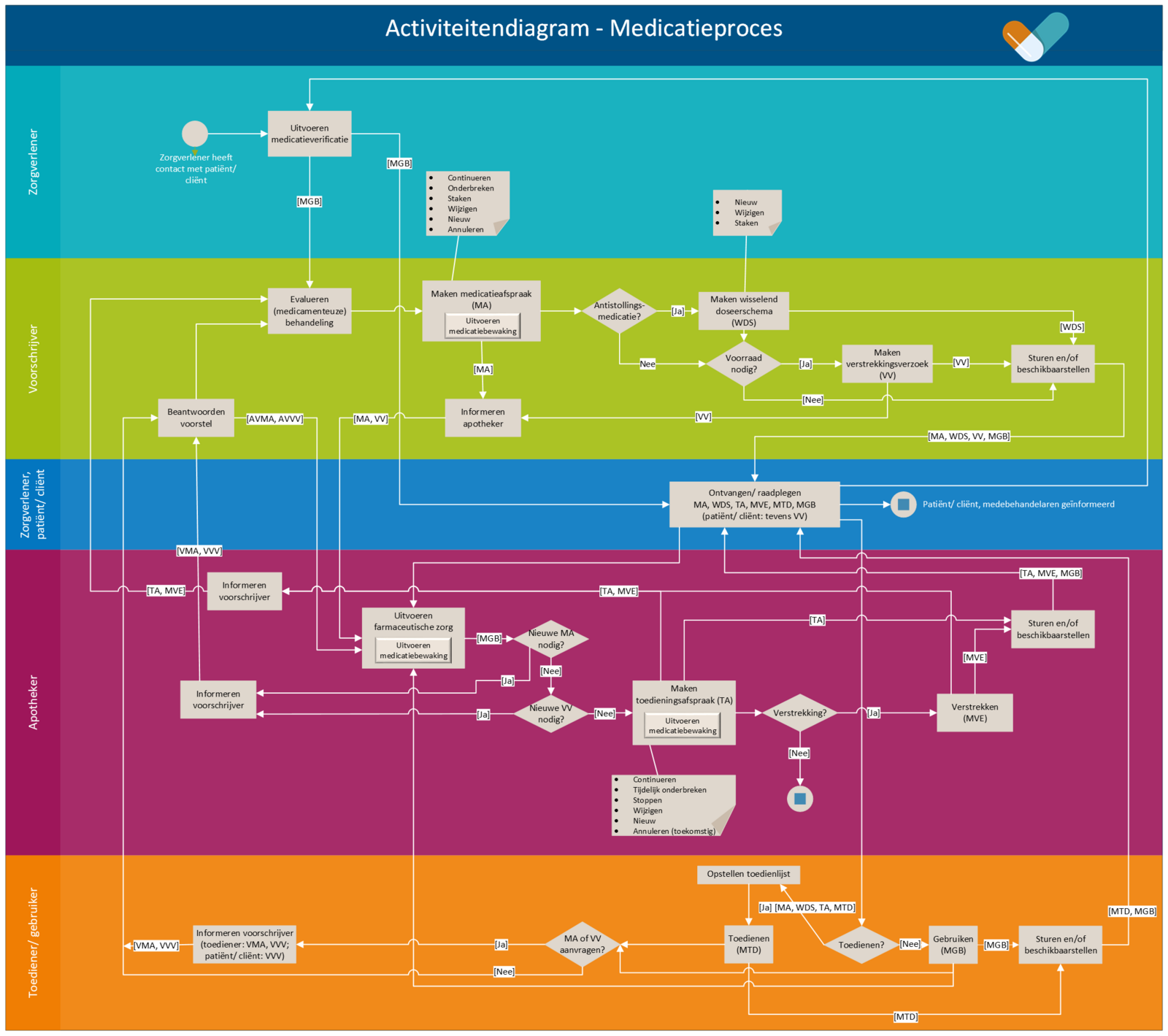
The medication process is a cyclical process consisting of prescribing, dispensing, administering and using medication. The process starts when the patient/client visits a health professional/healthcare provider (general practitioner, hospital or other institution) for a treatment with a medicinal product and ends when the medication is no longer needed. The process is depicted in Figure 4. The yellow bar indicates the medication verification process, green prescription, purple dispensing, and orange administration and medication use. The blue bar indicates receiving and consulting of data, which may take place in any of the subprocesses. This is described in further detail in the remainder of this chapter under the relevant subprocess. The following paragraphs describe the medication verification, prescription, dispensing, administration and medication use processes.
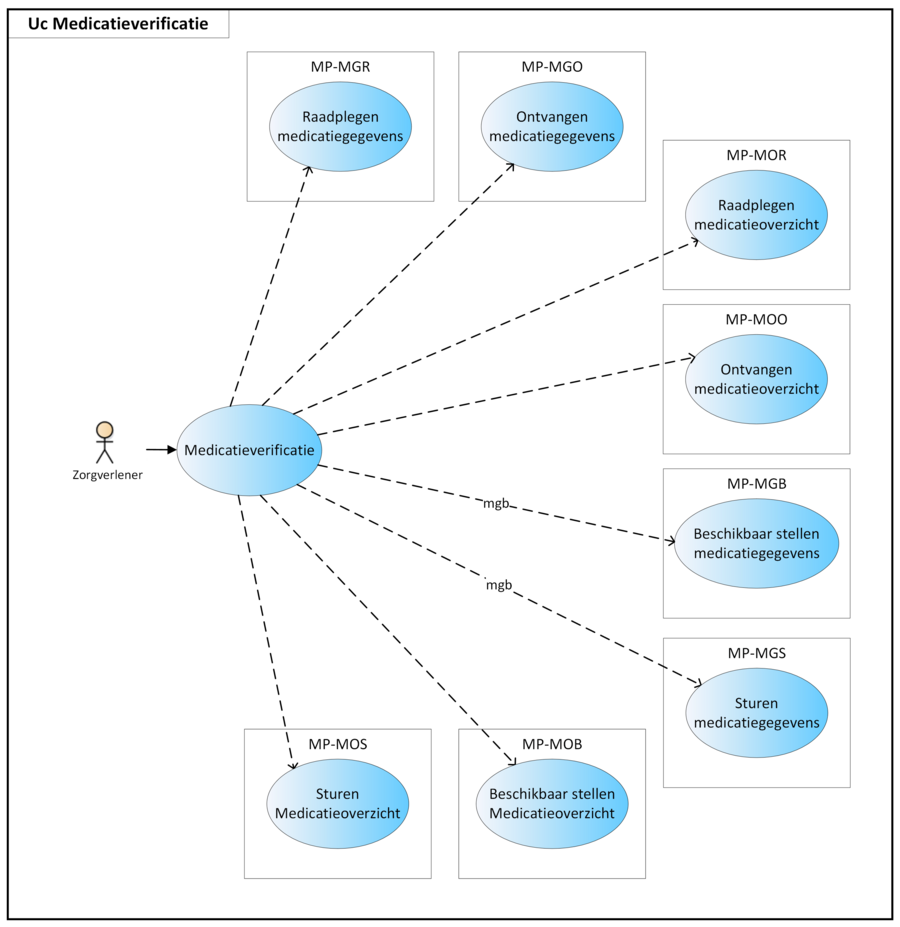
2.1 Process: medication verification
Prior to the prescription process, the patient’s actual medication use is determined. This is done[6]:
- In the GP practice by the general practitioner during a consultation,
- At the GP service, A&E department or mental health crisis service by the triage specialist or the treating physician, as soon as possible, upon arrival or admission,
- In case of clinical or day admission at a hospital or other institutions by for example the nursing staff, pharmacy assistant or outpatient/hospital pharmacist,
- In case of outpatient consultation by for example nursing staff, doctors’ assistant or the treating physician.
2.1.1 Current situation
- In the current situation, patients or family/informal caregivers are asked which medication they are using. The patient is sometimes unable to answer this. Family/informal caregivers (if known) are also often unable to answer this. If this is the case, the physician will contact the general practitioner or the pharmacist to find out the medication. This is difficult outside office hours and during weekends.
2.1.2 Precondition
The patient comes in for a consultation/an outpatient consultation or is admitted (in the future).
2.1.3 Trigger event
- Outpatient setting: consultation of and/or prescription to outpatients and patients residing in another healthcare institution[7]. In this case, medication verification often occurs during treatment assessment (see paragraph 2.2.4).
- Clinical setting: preparation of patient admission.
2.1.4 Process
The health professional collects the medication data from various sources, which may include:
- Patient’s own story,
- Dispense overviews from pharmacies,
- Digitally available medication data from healthcare providers or personal health records (PGO),
- Medication brought in by the patient,
- If necessary, information by telephone from the patient’s own pharmacist or general practitioner.
The health professional verifies the medication together with the patient and records the verified medication as MGB, incl. self-medication. This results in an updated medication profile; see also paragraph 5.6.
In practice, medication verification will lead to recording of MGB, only when it proves clinically relevant followed by updating the medication profile, particularly upon admission and discharge. The recorded data related to medication use are made available to fellow health professionals and the patient so that they can consult the data.
2.1.5 Postcondition
The patient's medication use has been recorded and medication data (MGB) have been made available.
2.1.6 Information systems and transaction groups
Chapter 7 includes an overview of all information systems, system roles, transactions, etc. Those most important for the medication verification process are included in the overview below.
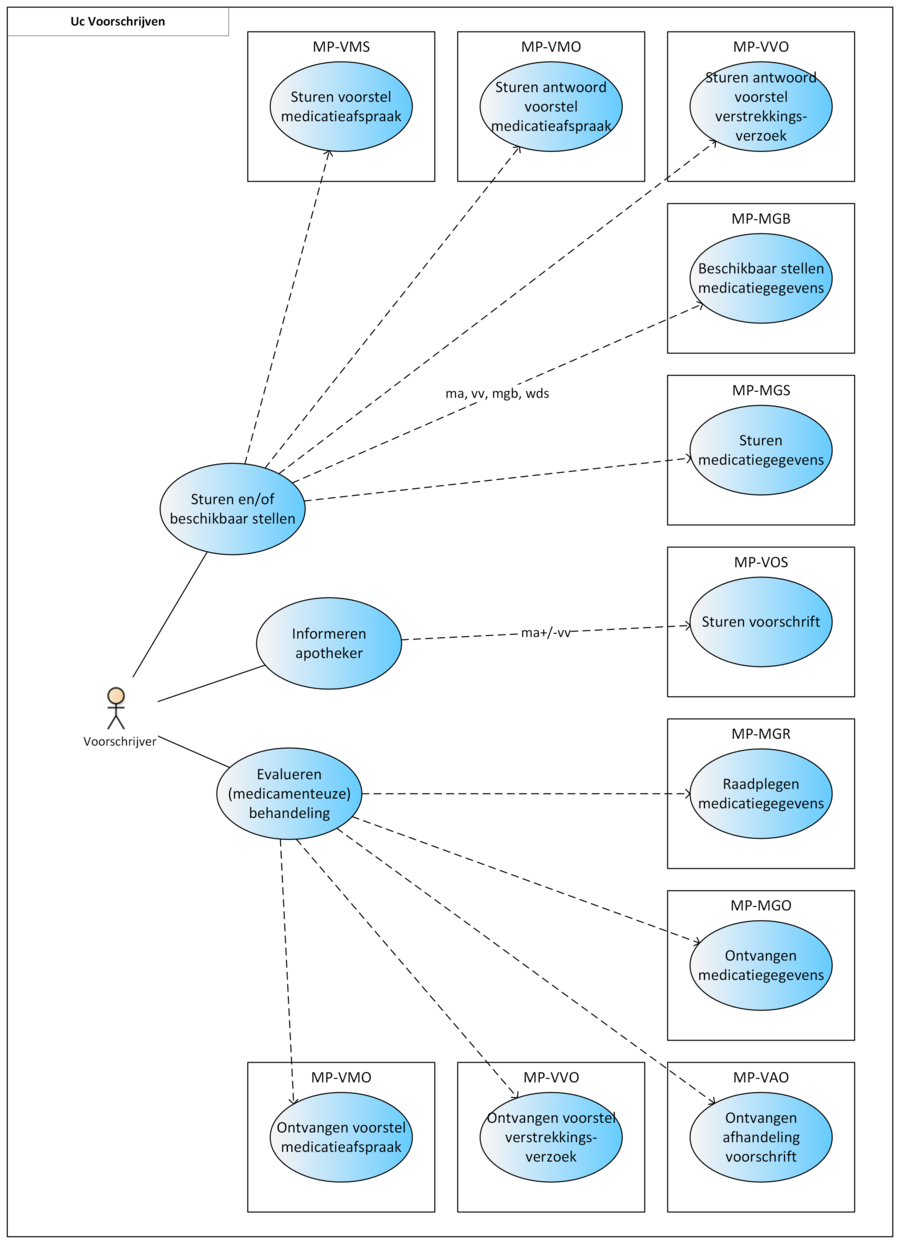
2.2 Process: prescribe
This section describes the process of prescribing. The role of prescriber may be performed by anyone with a prescribing authorisation according to the law BIG. In addition, a prescriber may delegate this task to other healthcare providers who do not have this authority themselves. This is only allowed within the terms of the law and the agreements within the relevant healthcare organisation.
The prescription process consists of an evaluation of existing pharmaceutical treatment, if any. If necessary, an MA is created and, only in an ambulatory situation, possibly a VV. Finally, the recorded medication data (MA, WDS, MGB, VV) are sent and/or made available. A picture of this process can be found in Chapter 2.
2.2.1 Current situation
The following deviations from the desired situation that are currently observed are:
- The logistical process often determines whether information is recorded (and certainly if it is communicated). Changes in medication or discontinuation are insufficiently recorded and/or communicated, resulting in, among other things, inaccurate monitoring, incorrect use and incorrect medication profiles.
The pharmacotherapeutic policy should be leading, not the logistical process as is currently the case.
- Since the therapeutic intention is not communicated to the pharmacist, it is not possible to deduce from the available data whether a request for a repeat prescription falls within that therapeutic intention. Because of this, use may be erroneously resumed or continued.
- If a change is not communicated, a request for a repeat prescription (through the pharmacist) may be based on outdated instructions for use. This can easily lead to errors.
- In an outpatient setting, MAs and/or VVs are usually not (electronically) sent to the pharmacist.
2.2.2 Precondition
There is a certain reason why a prescriber wants to start or evaluate/review a (pharmaceutical) treatment.
2.2.3 Trigger event
The trigger event for the process is the start of a new MBH, the evaluation of an ongoing treatment, receipt of a VVV or VMA, receipt of a notification of prescription processing from the pharmacist, or patient admission to or patient discharge from an institution.
2.2.4 Process step: Evaluating a pharmaceutical treatment
In order to evaluate treatment, an up-to-date overview of medication data is required. The medical file from the health professional is, where possible and if necessary, updated with data from external sources. In addition, the patient may be asked which medicinal products he is currently using. This medication use can be recorded by the health professional. If desired, a more extensive medication verification can be carried out (see paragraph 2.1).
The treating physician[8] evaluates the (pharmaceutical) treatment and decides to:
- start a new MBH by creating an initial MA and/or
- continue, discontinue, temporarily halt or modify an existing MA (1 or more)[9] and/or
- correct/cancel an existing MA and/or
- approve a VMA or VVV (reply via AVMA or AVVV is optional) and/or
- reject a VMA or VVV (reply via AVMA or AVVV is mandatory) and/or
- send a VMA to another prescriber.
These situations are further explained in the following paragraph. See also paragraph 1.3.3 for more information on the concept of MBH, and paragraph 2.3.5 for further information on managing proposals.
2.2.5 Process step: Making a medication agreement
When creating an MA, the following principle applies: each change is recorded in a new MA. Technically this means that the existing MA is terminated by entering an end date (hereafter: endDateTime according to naming convention in the dataset) and that a new MA is created with the desired changes[10].
An MA can also be created to begin at a point in the future. These MAs will receive a PeriodOfUse with a startDateTime in the future, which is later than the date of the agreement itself. Any prior MA will have an endDateTime just before the startDateTime of the future one. In the PeriodOfUse it is possible to only register a startDateTime without Duration or endDateTime. This is the case with continuous medication. To avoid confusion between 'to/until/till' and 'up to and including', specifying the time is mandatory when entering an endDateTime. In case of an 'up to and including' date (in case of an entire day), the time 23:59:59 applies.
Before the MA is sent and/or made available, medication monitoring takes place in accordance with current guidelines. This is a part of this process step.
The next paragraphs describe the different situations in which an MA is created, i.e. initial medication agreement, continuing medication, discontinuing medication, temporarily halting medication or correcting/cancelling an agreed medication. Information about MBH is assumed to be known (see paragraph 1.3.3).
2.2.5.1 New medication agreement
A new MA is created at the start or modification of an MBH. When a new MBH is started, the prescriber should consider whether an existing MBH should be discontinued. The description in paragraph 1.3.3 is based on the most common process from prescription to administering or using. In the hybrid situation or in the absence of digital data, it is also technically possible that an MBH could start with a TA, for example. This might occur for instance when a pharmacist has not received the MA with the corresponding MBH in digital form. The pharmacist will consequently start a new MBH when the TA is created. This may also be the case for any other building block. A patient can, for example, start an MBH by recording MGB, without having the original MBH.
2.2.5.2 Continuing medication
In a number of cases the therapeutic intention of the prescriber remains the same and the MA does not have to be modified. For instance:
- In an ambulatory situation when, for a repeat prescription, only a new VV is needed, or
- At admission to an institution where the home medication continues to be used, whether or not in combination with self-medication.
In both of these cases, the existing MA will not be adjusted. Should there be a change in PRK, e.g. at admission or discharge, the existing MBH will be discontinued by creating a stop-MA (see paragraph 2.2.5.3) and a new MBH is started (see paragraph 2.2.5.1).
2.2.5.3 Discontinuing medication
Medication is discontinued by creating a new MA (stop-MA) within the same MBH. The reason for discontinuation is recorded in this stop-MA, in data element ReasonModificationOrDiscontinuation. The medication may be discontinued immediately with endDateTime today, or stopped with endDateTime in the future. The new stop-MA contains the following information:
- An endDateTime (may also be in the future),
- Its own author,
- Its own RegistrationDateTime,
- MedicationAgreementStopType 'discontinued',
- Reference to the specific MA that is being stopped (future MAs will remain in place). It is not possible to create a stop-MA without referring to the MA that needs to be stopped except when that MA is not available. If there are only TA(s) or MGB(s) available in the MBH then a prescriber must be able to stop these with a stop-MA without reference to an MA. The stop-MA also gets no relation to a TA or MGB in this case, because the intention is to stop the entire MBH, not just that specific TA or MGB.
- ReasonModificationOrDiscontinuation.
From the referenced building block, at least the following data elements must be copied into the stop-MA:
- startDateTime,
- AgreedMedicine, with exception of 90 million numbers,
- InstructionsForUse, with exception of AdditionalInstructions.
For an MA in which an endDateTime has immediately been agreed upon, e.g. in the case of a course of treatment, no additional stop-MA is needed. A stop-MA also has the ‘discontinued’ stop type, even if it is a stop-MA resulting from a change. In case of a change, the stop-MA is followed by a new MA.
A stop-MA resulting from a change is not always relevant for end users. A stop-MA as a result of a change is also referred to as a technical stop-MA. The user interface must adequately support this. A prescriber will be less interested than a pharmacist who may need to adjust his logistical process because of the change. When a prescriber wants to shorten the PeriodOfUse even more after creating a stop-MA, another stop-MA is created. The stop-MA refers to the most recent MA and this is the first stop-MA.
Medication can be stopped by the prescriber himself or by another prescriber. When a prescriber stops medication he registers a new stop-MA, also when stopping someone else’s MA. He sends the stop-MA to the health care provider who made the original MA to notify him/her. The latter processes the stop-MA in his/her own system, if possible.
In some situations, MAs may unintentionally seem to be active when in reality this is no longer the case. To minimise this risk, information systems must, in response to a query, for each MBH provide the stop-MA with the most recent RegistrationDateTime, even if this building block falls outside the requested PeriodOfUse. This way, it is still possible to determine that a medication building block is not active anymore, but stopped.
2.2.5.4 Temporarily halting and resuming medication
Temporarily halting medication is the discontinuation of medication for a known or unknown period of time. A suspension may take effect immediately or a future suspension can be planned. When medication use is temporarily halted, the medication still remains relevant for monitoring because the medication may be resumed in the future. Temporary substitution with another medicinal product is not considered a suspension but rather a discontinuation of the original medication and the start of a new pharmaceutical treatment with the substitute.
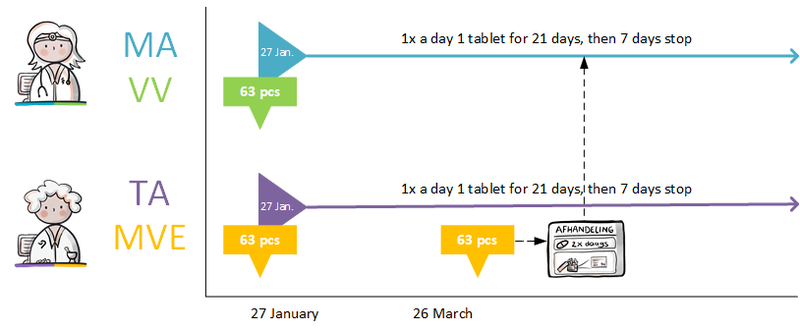
Temporarily halting medication is covered by two MAs[11]. A stop-MA is created to stop medication use in accordance with the guidelines for a stop-MA (see previous paragraph). The stop-MA has a relation to the original MA in order to temporarily stop that MA. The reason for the suspension is recorded in ReasonModificationOrDiscontinuation. The stop type of the stop-MA is 'suspended’.
A new MA is created for resuming the medication, including the reason for resuming, if any (in ReasonModificationOrDiscontinuation). All these MAs are part of the same MBH.
2.2.5.5 Changing medication
Changing an MA may apply to:
- Dosage,
- Method of administration,
- Duration of treatment ((e.g. extension of therapy),
- The responsible prescriber.
Changes in the dose strength or switching to a completely different medicine results in a different PRK thus switching to a different MBH (see also paragraph 1.3.3). In this case, the doctor will discontinue the existing MBH (see paragraph 2.2.5.3) and start a new one (see paragraph 2.2.5.1).
If the PRK stays the same, changes will be recorded under the same MBH. In case of a change, a technical stop-MA is made (see paragraph 2.2.5.3) and a new MA is made with the relevant change. Should the change apply to a future MA, a technical cancel-MA is made (see paragraph 2.2.5.7) and a new future MA is created with the relevant change. The RegistrationDateTime of the technical stop/cancel-MA and the new MA should always be the same. In the new MA a reason for the change is recorded in ReasonModificationOrDiscontinuation, and, if possible, a reference to the original MA. A change may take effect immediately or can be scheduled with a startDateTime in the future. A technical stop-/cancel-MA and corresponding new MA are made available at the same time.
Shortening an MA's PeriodOfUse is considered a change. A stop-MA needs to be made and a new MA2 with the shortened PeriodOfUse.
A stop-MA cannot prolong the PeriodOfUse. Extending an MA can be done in the following ways:
- Creating a new MA2 with a startDateTime after the endDateTime of the original MA1. There is no need for a stop-MA on MA1, as this expires automatically on its endDateTime. The new MA2 can be created both during and after the PeriodOfUse of MA1.
- Extending the PeriodOfUse of the original MA1 if it has not yet expired. This is a common change where a technical stop-MA is made and a new MA2 with the extended PeriodOfUse.
2.2.5.6 Correcting a medication agreement[12]
This paragraph describes correcting an MA because a prescriber made an error. This may have been discovered by the prescriber himself or by a co-prescriber. For example, a doctor makes a typing error in the dosage of an MA: 10 inhalations, 2x daily, instead of 1 inhalation, 2x daily.
If the MA has not yet been shared with other healthcare providers, the prescriber can modify or delete that MA himself within his own information system.
If the incorrect MA has already been shared with other healthcare providers, then he will stop this erroneous MA with a stop-MA with the reason 'incorrect registration' (in ReasonModificationOrDiscontinuation) and create a new MA under the same MBH with the correct information.
Should the erroneous MA have a startDateTime in the future, the prescriber cancels it with a cancel-MA (see paragraph 2.2.5.7) with the reason 'incorrect registration'. The doctor sends the stop/cancel-MA and new MA to the pharmacist and makes them available to fellow health professionals and the patient. (see paragraph 2.2.10).
2.2.5.7 Stopping a future medication agreement
This concerns only MAs with a startDateTime in the future. A prescriber may wish to terminate this future MA for any reason. Instead of being stopped, the future MA is cancelled, making it clear that there has been no PeriodOfUse.
The recognisability of a cancel-MA is necessary, e.g. for it to be processed correctly when preparing a medication summary. It is of course important for a healthcare provider to know whether something actually took place and then was stopped, or that it never took place. Distinguishing the cancel-MA and stop-MA makes this transparent.
The cancel-MA basically works the same as the stop-MA described in paragraph 2.2.5.3, but with two important differences:
- The stop type is 'cancelled' instead of 'discontinued'
- The startDateTime and stopDateTime of the cancel-MA are the same.
2.2.6 Process step: Making a variable dosing regimen
When a prescriber prescribes medication with a variable dosing regimen (WDS), the dosing of the medication can be adjusted by a (different) prescriber without having to adapt the MA. At the moment the WDS is used for anticoagulants. When prescribing anticoagulants, the prescriber determines the therapeutic INR-range (International Normalized Ratio, a measure of blood clotting time), within which the treatment should take place.
The thrombosis service is responsible for drafting the WDS. The dosing regimen is entered in the medication building block WisselendDoseerschema (WDS) by a prescriber, usually the thrombosis physician. . The prescriber who made the MA remains responsible for the VVs. The thrombosis physician composes the WDS within the agreed upon INR-range based on a specific, measured INR-value.
2.2.6.1 Setting up a variable dosing regimen
The prescriber prescribes anticoagulants with an MA and indicates:
- The medication (PRK) that is being prescribed to the patient. The medication in the WDS is always the same as the medication in the MA.
- That for this medication a variable dosing regimen applies (this is added in the additional instructions). This also means there will be no dosage added in the MA.
- The INR-range within which the treatment should take place. This information is included in a comment.
In order to bridge the period until the thrombosis service is involved and ready to take over the treatment, the original prescriber sets up a WDS for this first period (usually between 4 and 7 days). Usually a checkup date is agreed upon after the registration of the patient at the thrombosis service. During this checkup the INR-value is measured. Based on the measured value and/ or the professional assessment of the thrombosis physician, a dosing regimen is composed that either changes of succeeds the previous WDS. From this moment on, the thrombosis service takes over the composition of the dosing regimen from the original prescriber.
INR-value with the WDS
The WDS is often based on an INR-value. For other parties involved in the care for the patient, it’s important to be able to deduce the INR-value on which the WDS was based. For this reason, the corresponding INR value in the WDS can be recorded in free text in the 'comment' data element.
2.2.6.2 Changing a variable dosing regimen
When the WDS is being used up until its endDateTime, it can be replaced by a new WDS that succeeds it. The new WDS has a relation to the MA and the previous WDS. It is also possible to adjust the WDS before the endDateTime has been reached. In that case, the information system stops the previous WDS, using a technical stop-WDS that is not visible to the user. The new WDS follows and has a reason for change and a relation to the MA and the previous WDS. All changes related to the dosing regimen can be included in the WDS. Changes that concern the further treatment policy (e.g. the prescribed medication, the route of administration of the agreed upon INR-range) are to be made in the MA.
2.2.6.3 Stopping a variable dosing regimen
There are various reasons that can cause the need to (temporarily) stop the use of anticoagulants. For example, in the event of a procedure or because there is temporary co-medication. Two kinds of situations can be distinguished. Depending on the situation and the assessment of the thrombosis physician one of twee methods is chosen:
- (temporarily) adjusting the policy: The thrombosis physician can choose to temporarily adjust the dose for the anticoagulants to 0. For example, in the event of a planned procedure. In this case, the MA (and therefore the treatment with anticoagulants) and the TA will continue, but the dosage in the WDS is temporarily adjusted to 0. The MA will still be shown on the medication overview under ‘current medication’. In the event of such a temporary adjustment, only the WDS is changed. It is advised to include a reason for this change in the new WDS.
- (temporarily) stopping the anticoagulants: The thrombosis physician (or another prescriber) can also choose to (temporarily) stop the treatment with anticoagulants. This means that the patient should not take any anticoagulants anymore. In this case the thrombosis physician (or another prescriber) creates a stop-MA (or sends a VMA to the original prescriber). Stopping the MA also stops the underlying therapeutic building blocks (TA, WDS). The anticoagulant will be shown on the medication overview under ‘recently stopped medication’.
If, after a period of time, there is a need to restart the anticoagulant, the prescriber can do this by creating a new MA. In many cases the thrombosis service will no longer be involved. But if they are, the thrombosis physician could send a VMA to the original prescriber who then may adopt the proposal and create an MA accordingly.
Anticoagulant medication can be stopped by the prescriber himself or by another prescriber. When a healthcare provider stops anticoagulant medication he registers a stop-WDS, also when stopping someone else’s WDS. He sends the stop-WDS to the healthcare provider who created the original WDS to notify him/her. The latter processes the stop-WDS in his/her own system, if possible.
In some situations, the WDS may unintentionally seem to be active when in reality this is no longer the case. To minimise this risk, information systems must, in response to a query, for each MBH provide the stop-WDS with the most recent RegistrationDateTime, even if this building block falls outside the requested PeriodOfUse. This way, it is still possible to determine that a medication building block is not active anymore, but stopped.
2.2.7 Process step: Creating a dispense request
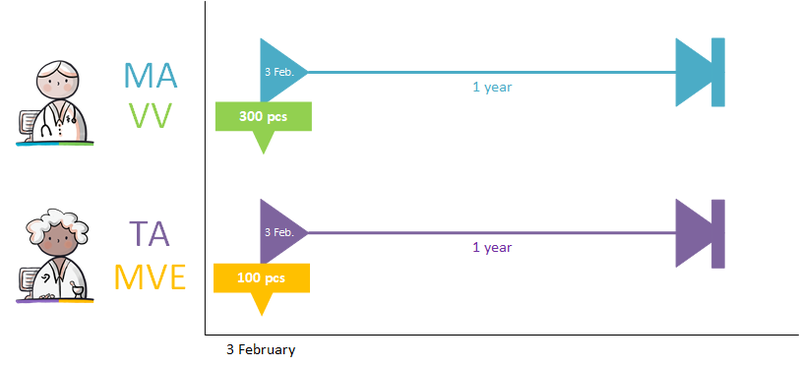
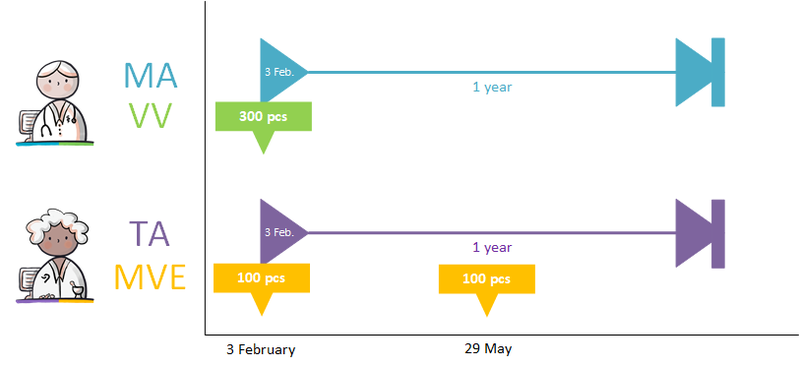
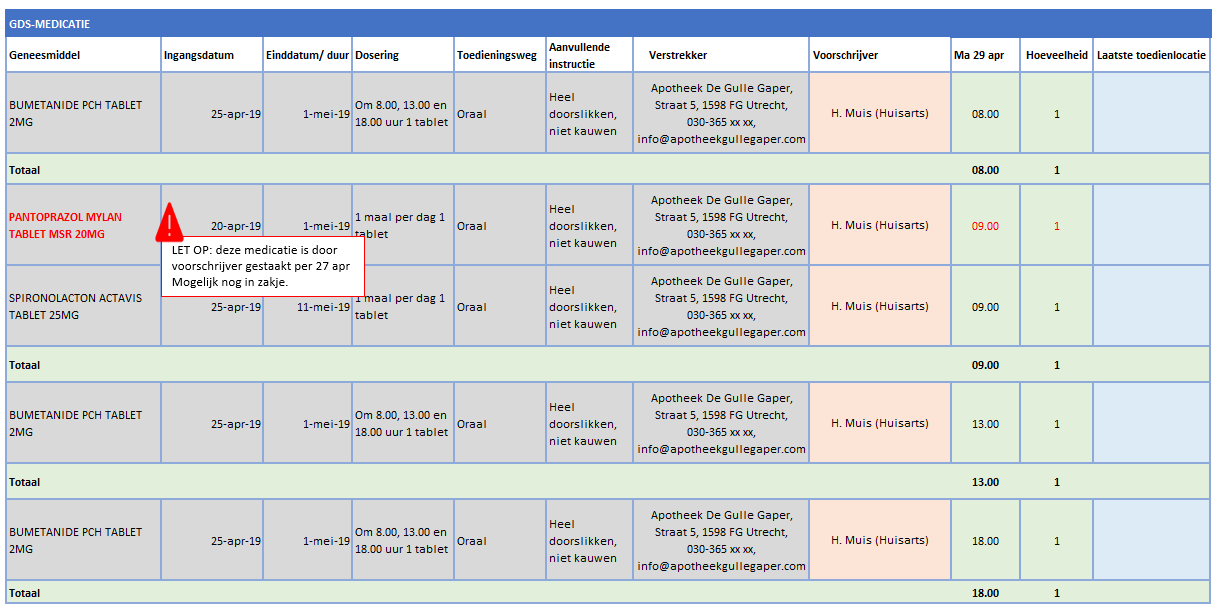
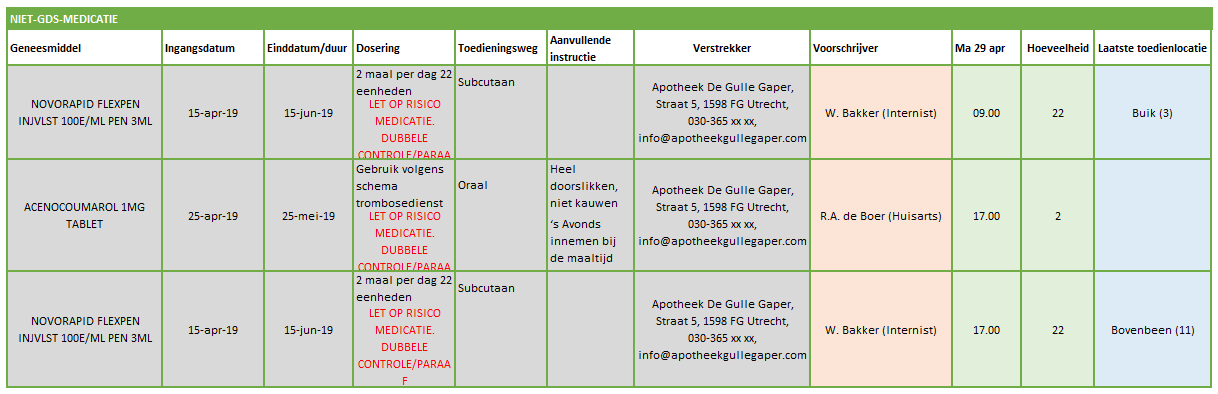
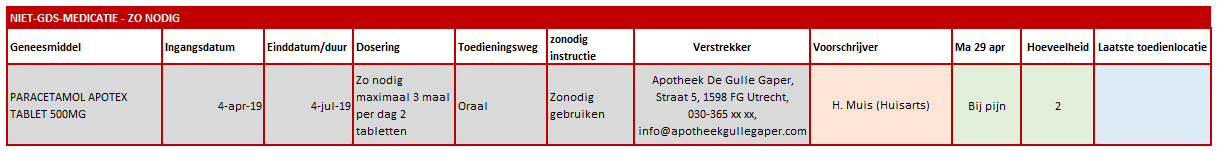
A dispense request (VV) besides an MA only applies in an ambulatory situation. A VV may be made when the medication supply of the patient needs to be replenished. This does not have to coincide with an MA. At the start of an MBH for which the patient still has a sufficient supply at home from a previous agreement, a VV is not needed. When the dosage is reduced, the patient may also have a sufficient supply. In the case of a medicinal product that is used for a prolonged period of time (e.g. an antihypertensive drug), with a continuous MA (meaning a PeriodOfUse with only a startDateTime), several VVs may be made over time within the scope of this existing agreement. It is also possible for a prescriber other than the one who made the MA to make a VV under this MA. Logistical and emergency instructions may be included in the VV, such as supply location, request to not include in the GDS (medication distribution system, in Dutch: 'Geneesmiddel Distributie Systeem'), etc.
In the case of a VV, the quantity to be supplied (Amount) or the consumption period (PeriodOfUse) can be stated. In case of a consumption period, the quantity must be clearly deducible from the dosing instruction of the MA. Note: a consumption period endDateTime has a meaning other than the PeriodOfUse endDateTime from the MA and may diffeer.
- VV PeriodOfUse endDateTime: date until which the pharmacist is allowed to supply medication (and thereby provide sufficient supplies to the patient for use until that date).
- MA PeriodOfUse endDateTime: date on which the patient must stop the medication (this can be equal to the consumption period endDateTime or lie further in the future).
2.2.8 Process step: Sending renal function value with prescription
Renal function is important for certain medicines. The renal function value determines the choice of drug and/or drug dosage. It is legally stipulated that if a health professional has performed further research on a patient renal function, he should share abnormal renal function values with the appropriate pharmacist, appointed by the patient (article 6.10, 'regeling geneesmiddelenwet' - Dutch medicines act).
The renal function value is always sent with the prescription (using the building block laboratory test results) for medicines for which this is important, so that the pharmacist can perform proper medication monitoring. The renal function value should not be older than 13 months, because with stable chronic renal impairment the renal function should be checked at least once a year. 1 month has been added to allow some backlog in practice.
If at the time of sending of an MA and/or VV no renal function is known, then the prescription policy remains unchanged. Sending the laboratory test result renal function value without an MA and/or VV is beyond the scope of this information standard.
2.2.9 Process step: Sending height and weight values with prescription
It is possible for the prescriber to send the body height and weight of the patient with the prescription. This is the height and weight of the patient that the prescriber used for the MA. Hence, it is possible for these values to diverge (be more accurate) from the height and weight values that are registered in the patient details. This could be necessary for prescribing for children or for medication where weight (e.g. coagulation medication) or height (e.g. oncolytic agents) are important factors to consider.
Body height and weight are separate building blocks. They can only be sent together with the prescription and with the MA. The information is not available on request.
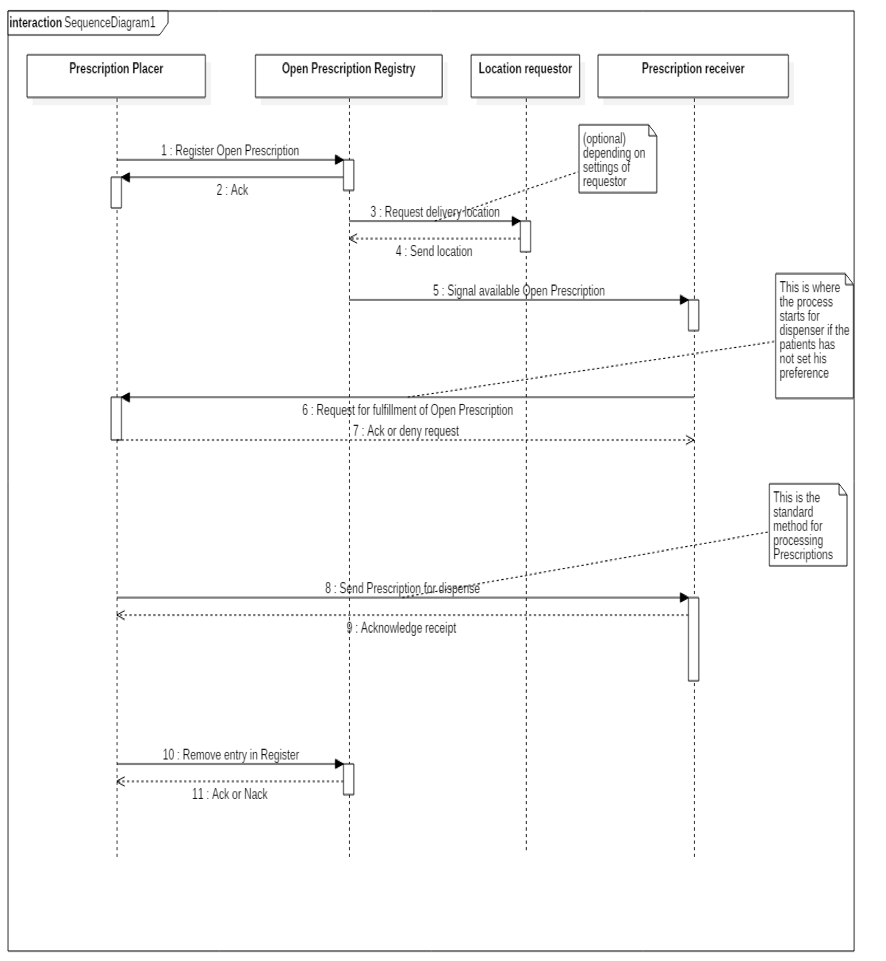
2.2.10 Process step: Send and/or make available
This step involves information exchange. Information can be sent or made available with different intentions:
A. As an order for the pharmacist to dispense medication. The prescriber sends the MA to the pharmacist. In an ambulatory situation, the VV is also sent to the patient’s pharmacist. If the pharmacist is not known, this process step can also be performed by making the data available (see C). When the pharmacist has filled the order, the prescriber will receive a notification (see paragraph 2.3.10).
B. As an order for the pharmacist to implement a medication change (including stop-MA with stop type 'discontinued' or 'suspended') that impacts or may impact a current dispense process by this pharmacist. A current dispense process indicates an order (as in A) that has been accepted, but has not yet been completely filled. For example, when medication is still being supplied or when a VV dictates that medication should be supplied multiple times, but not all supply actions have taken place yet, e.g. with the GDS.
C. Making available the medication data (MA, WDS, MGB; VV only to the patient), so that fellow health professionals and/or the patient can consult them at a later date.
D. Sending medication data to another healthcare provider at the patient's request or upon discharge.
E. Sending medication data (WDS) to the thrombosis service in case the prescriber has created an initial WDS before the thrombosis service takes over (see paragraph 2.2.6.1).
In case of corrected data, the prescriber assesses who should be actively informed about this correction. This can be done, for example, by sending the new MA (option A or B above) or by means of a telephone consultation.
2.2.11 Postcondition
- A new MA may have been created (starting, changing or discontinuing medication)
- A VV may have been made (only outpatient)
- An order may have been sent to the pharmacist to carry out a dispense of medication
- An order may have been sent to the pharmacist to modify a dispense of medication
- The new medication data (MA, WDS, MGB, VV) have been sent and/or made available to fellow health professionals and the patient.
2.2.12 Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[13]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
2.2.13 Practical examples
The following specific practical examples have been elaborated:
- Short-term medication
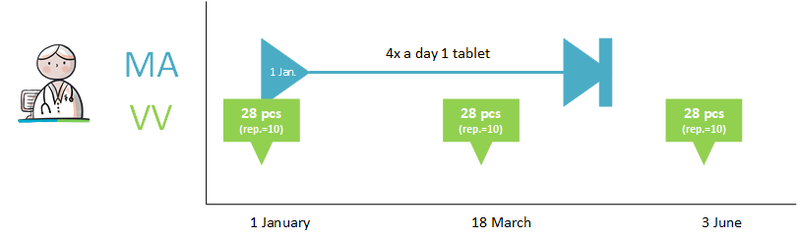
- Continuing medication
- Hard endDateTime for PeriodOfUse
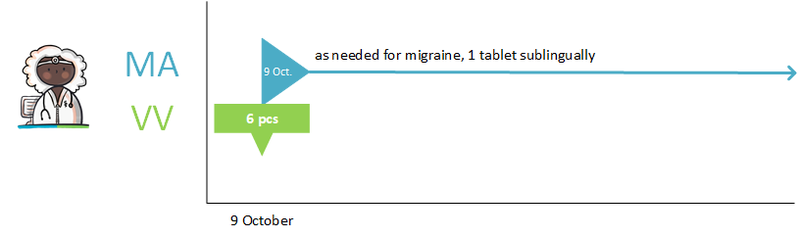
- Medication as needed
- Course of treatment as needed starting in future
- Two dosages of the same medication at the same time
- The same medicinal product with different strengths at the same time
- Extra particularities in the medication agreement
- New medication agreement, no dispense request
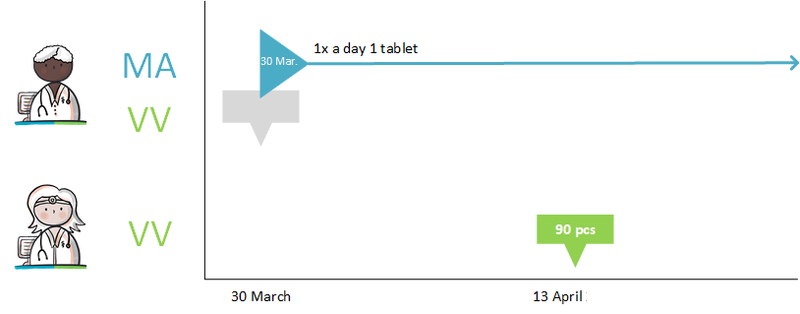
- New dispense request under existing medication agreement
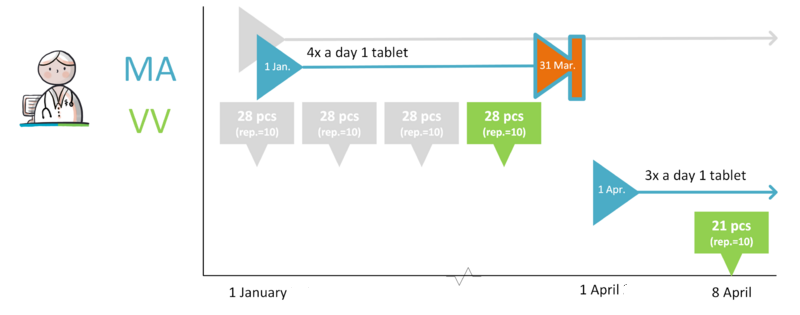
- Dosage change (sufficient supply)
- Prescription no longer needed after first dispense request
- Discontinuing medication
- Temporarily halting/resuming medication
- Temporarily halting for an intervention
- Paper prescription - no more allowed
- Carrying out medication verification and evaluation of foreign or self-medication
- Day treatment
- Starting with medication before admission
- Emergency admission
- Interim discharge
- Transfer to another institution
- Do not dispense before
- Discontinuation of medication by third parties
- Two PRKs in a single pharmaceutical treatment
- Creating a medication agreement after the fact
- Single medication use
- Provisional and final medication order
- Inadvertently ‘outstanding’ medication or 'orphans'
- Missing digital medication agreement at admission
- Own articles (90 million numbers)
- Dosing with minimum interval
- Dispense request with number of repetitions
- Prescribing non-medicines (paragraph 4.1.37)
- Send renal function value in the prescription
- Cancelling a prescription that was sent earlier
- Modification of someone else's medication agreement
- Setting up a variable dosing regimen
- Changing a variable dosing regimen during period of use
- Stopping medication with a variable dosing regimen
- Patient requests repeat prescription via physician (reactive repeat)
- Merging building blocks under one MBH
- Changing someone else's MA by non-prescriber with delegated responsibility
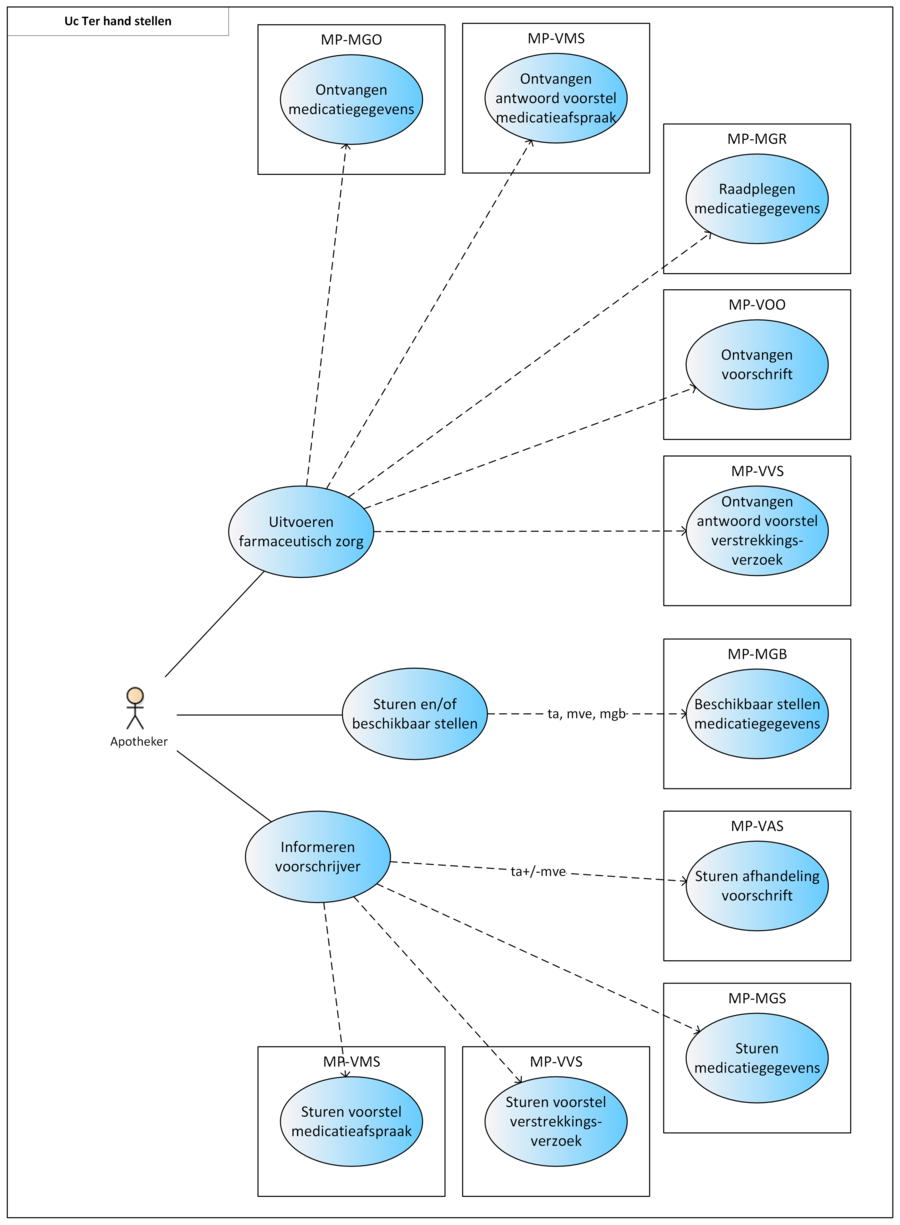
2.3 Process: dispense
This paragraph describes the process of dispensing medication, including repeat prescriptions and GDS. This process encompasses all actions a pharmacist must take for the patient to not only receive a medicinal product, but to also receive the associated pharmaceutical care ensuring a safe and effective use of the medicinal product by the patient. The dispense process starts with providing pharmaceutical care. If necessary, a TA will be made and, if needed, medication is supplied. Medication supply (i.e. handing out a medicinal product) does not always take place. This may be the case when the MA is changed (e.g. in case of dose reduction where the patient still has enough supply), when the medication is discontinued or, in an ambulatory situation, the medication is not collected. When the MA and/or the VV do not comply (see paragraph 2.3.5), the prescriber will be informed. Finally, the recorded data (TA, MVE) are sent and/or made available.
Medication assessment is the process in which the physician and pharmacist consider all medication of the patient against the background of his condition, applicable treatment guidelines, the well-being of the patient, etc. Medication assessment is defined in this document as a combination of treatment evaluation (as described in the previous paragraphs) and pharmaceutical care. Depending on the findings, the previously described medication verification and prescription processes are followed, after which medication dispense takes place.
For the GDS, many pharmacists collaborate with another organisation that handles part of the pharmacists’ logistics. This then also requires data exchange with that party. Besides, not all medication can be included in the GDS packaging, which adds to the logistical complexity for the pharmacist. The internal logistics and communication between the pharmacist and his subcontractor(s) are outside the scope of this information standard. It has been established however that with the provision of the current building blocks and the underlying data elements, this logistical process can be adequately supported.
2.3.1 Current situation
The following deviations (from the desired situation that) are currently observed:
- In the current situation, in the case of GDS (medication distribution system, Dutch: 'Geneesmiddel Distributie Systeem'), the prescribing physician and other health professionals receive a large quantity of dispense messages. This becomes difficult to manage for these health professionals.
- In the current situation, in the case of GDS, pharmacists and general practitioners communicate via so-called authorisation lists. The pharmacist sends an overview of all the patient’s medication to the general practitioner for authorisation. This complicates matters for a general practitioner because he will need to verify all MAs. This should not be necessary, as most MAs/VVs have already been authorised. It would be much more efficient if the general practitioner only needs to authorise those MAs/VVs that have not been authorised yet, e.g. a new VV on the basis of an existing MA.
- In the current situation, an after-hours pharmacy often does not inform the regular general practitioner and regular pharmacy when medication is dispensed.
- In the current situation, a proposal for an MA is usually coordinated with the prescriber by telephone, and/or the pharmacist and prescriber have made an agreement about handling a warning from the medication monitoring system.
- The intention of the return message is not always clear: no distinction can be made in the return/delivery message between displaying a physical delivery and a transmission of information about a medication change.
- In the current situation, pharmacists sometimes register (and communicate) medication dispenses when they are preparing the medication for the patient instead of at the time when the medication is actually supplied to the patient. This means that medication that has not been collected is sometimes erroneously registered as medication dispense.
2.3.2 Precondition
An MA exists. In an ambulatory situation there may also be a corresponding VV.
2.3.3 Trigger event
The pharmacist starts the medication dispense process on the basis of one of the following events:
- Receipt of an order to make an MVE on the basis of a new MA. In an ambulatory situation, this order is always accompanied by a VV.
- Receipt of an order to process a new MA in an ongoing MVE.
- Receipt of a trigger (for example, via a patient or a repeat module of the pharmacist information system) for a repeat MVE under an existing or newly proposed VV.
2.3.4 Process step: Providing pharmaceutical care
Pharmaceutical care is provided by a community, outpatient (i.e. at the hospital) or institutional pharmacy, depending on the health professional who has created the MA:
- MA, possibly with a VV from the general practitioner or specialist: care provided by a community or outpatient pharmacy.
- MA from specialists and other prescribers in hospitals/institutions: care provided by an institutional pharmacy or a community pharmacy that supplies the respective institution.
Medication monitoring is also part of pharmaceutical care.
Based on the received MA or a change in the situation of the patient, the pharmacist decides how to apply this by:
- Making one or more new TAs.
- Continuing, permanently discontinuing, temporarily halting or modifying an existing TA.
- Rejecting the MA.
- Proposing a new MA.
- Proposing a new VV.
The last three situations are explained in the following paragraph. The first two situations are explained in paragraph 2.3.6. In conclusion of provided pharmaceutical care, which may comprise new agreements and medication dispenses, a new up-to-date medication overview may be compiled and made available.
2.3.5 Process step: Contacting the prescriber
There are a number of situations where the pharmacist contacts the prescriber, such as:
- When a new or modified MA is needed
- When a new VV is needed
A new or modified MA is needed:
- When a new TA cannot be created based on the received MA because the pharmacist suspects an error in the MA, or
- After a signal from the medication monitoring system as part of pharmaceutical care. The signal may indicate, for example, that the dosage should be lowered or increased, that it is advisable to select another medicinal product, that a product should be temporarily discontinued, that another additional product should be added, etc., or
- Based on medication use as reported by the patient during pharmaceutical care, or
- When the temporarily halted pharmaceutical treatment may be resumed.
In these situations, a TA is not yet created or modified. The pharmacist first contacts the prescriber to discuss the possible error and suggest an alternative. The pharmacist may also send a proposed medication agreement (VMA) to the prescriber. He recommends a specific MA in this proposal, together with the reason and arguments for that recommendation. If the prescriber approves the VMA, he changes it into a final MA. Sending an AVMA is optional as the new MA suffices as an answer. If the prescriber rejects the proposal an AVMA must be sent to the requester (see also paragraph 2.2.4 ff.).
A new VV is needed when the patient’s medication stock is depleted or nearly used and the treatment may need to be continued (request repeat prescription). The patient either requests a repeat MVE from the pharmacist or has signed up in the past for proactive repeat MVE and a notification signal is generated by the repeat module of the AIS when the patient requires new medication[14]. If the existing dispense request is not adequate, the pharmacist can send a VVV to the prescriber. The VVV may contain indications for the prescriber, such as urgency. If the prescriber approves the VVV, he changes it into a final VV. Sending an AVVV is optional as the new VV suffices as an answer. If the prescriber rejects the proposal an AVVV must be sent to the requester (see also paragraph 2.2.4 ff.).
2.3.6 Process step: Creating an administration agreement
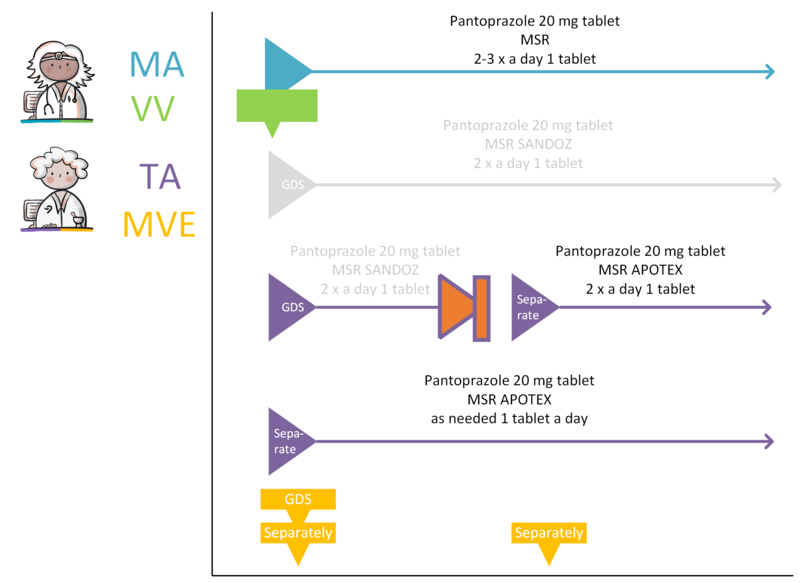
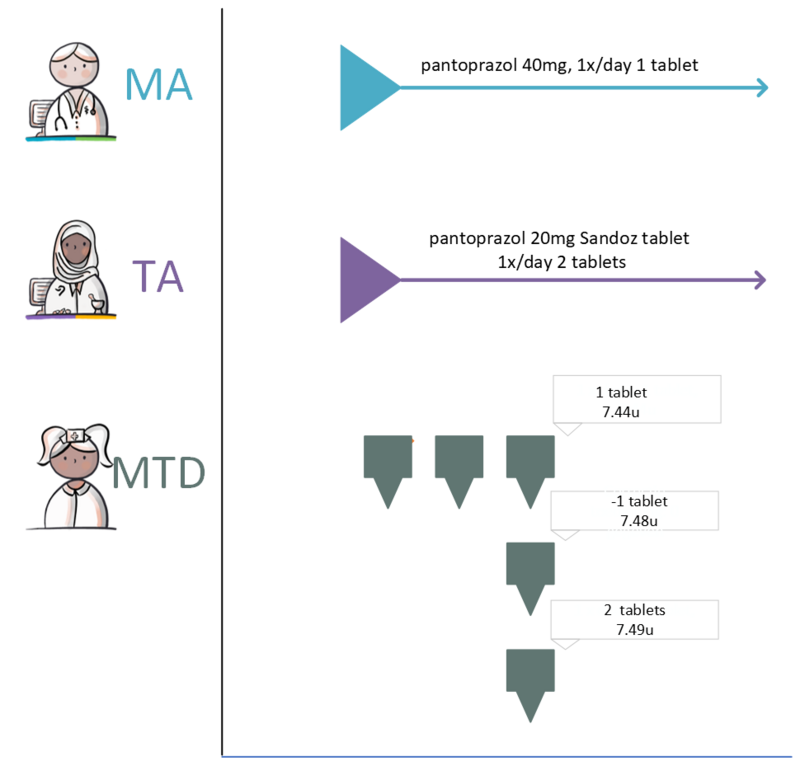
If the MA and, if applicable, the corresponding VV can be processed, a TA will be created. By creating a TA, the pharmacist fulfils the MA. The TA is communicated to the patient or the person administrating the medication. The TA belongs to the same MBH as the MA it fulfils. As is the case with the corresponding MA, a TA may start in the future. The dosage in the TA may deviate from that in the MA, for example because a certain strength is not in stock. This means that a drug with a different PRK may fall under the same MBH, for example, when changing from 1x 20 mg to 2x 10 mg tablets, Before the TA is sent and/or made available, medication monitoring will take place in accordance with applicable guidelines as part of this process step.
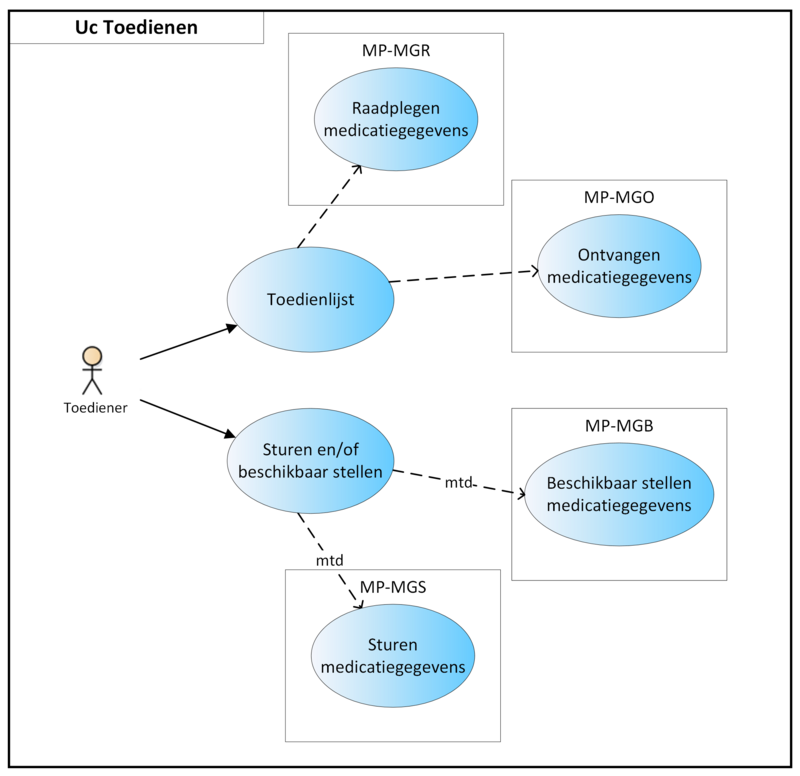
On the basis of the TA, an administration list[15] can be compiled for home care or nursing staff, among others.
When creating a TA, the same principle applies as for the MA: each change is recorded in a new TA.
The following paragraphs describe the different situations in which a TA is created: new TA or continuing, permanently discontinuing, temporarily halting or modifying an existing TA.
2.3.6.1 New administration agreement
In case of a new MA, a new TA is always created. A new TA is also created in case of a new preference policy or a change in stock which results in the selection of a different medicinal product. When creating a new TA, the pharmacist takes into account, among other things:
- Preference policy,
- Inclusion in GDS-packaging,
- Available stock of the institution (‘hospital formulary’) or the pharmacy itself.
Administration agreement for anticoagulants
Medication with a variable dosing regimen also requires a TA and/or MVE. This follows the regular process. However, the TA, like the MA, does not include a dosing schedule, but the additional instruction: 'use according to schedule thrombosis service'. See paragraph 2.2.6 for more information on the variable dosing regimen.
If, in an ambulatory situation, the first supply to the patient occurs later than agreed, the startDateTime of the TA will be different from the startDateTime of the MA.
A new TA can also be created without corresponding MA. When a patient buys over-the-counter medication in the pharmacy, the pharmacist may want to register this as a TA for the purpose of medication monitoring.
2.3.6.2 Continuing an administration agreement
When the existing MA and TA are sufficient to carry out an MVE, the TA will not be adjusted.
2.3.6.3 Discontinuing an administration agreement
A TA is discontinued by creating a stop-TA within the same MBH. The reason for discontinuation is recorded in this TA, in data element AdministrationAgreementReasonModificationOrDiscontinuation. The TA may be discontinued immediately with endDateTime today, or stopped with endDateTime in the future. The new stop-TA is a copy of the existing TA with:
- As endDateTime in the PeriodOfUse the date on which the TA ends (may also be in the future),
- Its own author,
- Its own RegistrationDateTime,
- Stop type 'discontinued',
- Reference to the specific TA that is being stopped (other TAs will remain in place). It is not possible to create a stop-TA without referring to the TA that is being stopped, except when there is no TA available in the MBH to refer to. If there are only MGB('s) available in the MBH then a pharmacist must be able to stop these with a stop-TA without reference to a TA.
For a TA in which an endDateTime has immediately been agreed upon, e.g. in case of change of roll (GDS), no additional stop-TA is needed. When a TA with an endDateTime in the future is being extended, this will be considered as a normal change (see paragraph 2.3.6.5). A stop-TA has the ‘discontinued’ stop type, too, if it is a stop-TA resulting from a change.
Also, an MA in which it has been agreed to discontinue medication permanently leads to a stop-TA with stop type ‘discontinued’ under the same MBH (this also applies in case of a stop-MA as a result of a change). The stop-TA prevents further supplying of the discontinued medication.
In an ambulatory situation, the prescriber can indicate in the MA that the medication will be discontinued starting with the next roll (with GDS). In this case, the startDateTime of the stop-TA may be later than indicated in the original stop-MA.
A TA can be stopped by the pharmacist himself or by another pharmacist. When a pharmacist stops medication, he creates a stop-TA, also when stopping someone else’s TA. He sends the stop-TA to the healthcare provider who made the original TA to notify him/her. The healthcare provider of the original TA then processes the stop-TA in his/her own system if possible.
In some situations, administration agreements may unintentionally seem to be active when in reality this is no longer the case. To minimise this risk, information systems must, in response to a query, for each MBH provide the stop-TA with the most recent RegistrationDateTime, even if this building block falls outside the requested PeriodOfUse. This way, it is still possible to determine that a medication building block is not active anymore, but stopped.
2.3.6.4 Temporarily halting an administration agreement
Temporarily halting an MA results in a stop-TA (stop type: 'suspended'). Upon resuming, a new TA is created. Both TAs belong to the same MBH as the MA.
2.3.6.5 Modifying an administration agreement
Changing a TA can take place on two different levels: by changing the corresponding MA or by changing the TA. Changing the corresponding MA (consisting of a technical stop-MA and a new MA) results in a stop-TA with reference to both that stop-MA and to the original TA. A new TA is also created for the newly created MA.
When the change is made by means of a technical cancel-MA and a new future MA, basically the same process applies. The corresponding TA is cancelled by a cancel-TA with reference to the cancel-MA and to the original TA. With the new future MA, a new future TA is created with reference that new MA.
If the change is made only to the TA, this will result in a technical stop-TA and a new TA under the existing MA. If the change is made to a future TA, a technical cancel-TA is created (see paragraph 2.3.6.6) and a new future TA with the change. The RegistrationDateTime of the technical stop/cancel-TA and the new TA must always be the same. The new TA should include the reason for the change (in AdministrationAgreementReasonModificationOrDiscontinuation) and, if possible, a reference to the original TA as well as to the unchanged MA. A change may take effect immediately or can be scheduled with a startDateTime in the future. A technical stop/cancel-TA and corresponding new TA are made available simultaneously.
2.3.6.6 Stopping future administration agreement
This concerns only TAs with a startDateTime in the future. A pharmacist may wish to terminate this future TA for any reason. Instead of being stopped, the future TA is cancelled, making it clear that there has been no PeriodOfUse.
The recognisability of a cancel-TA is needed e.g. for it to be processed correctly when preparing a medication summary. It is of course important for a healthcare provider to know whether something actually took place and then was stopped, or that it never took place. Distinguishing the cancel-TA and stop-TA makes this transparent.
The 'cancel-TA' basically works the same as the stop-TA described in paragraph 2.3.6.3 but with two important differences:
- stop type is ‘cancelled’ instead of ‘discontinued’
- startDateTime and endDateTime of the cancel-TA are the same
2.3.7 Process step: Supply
After creating the TA, the pharmacist prepares the product and supplies it to:
- The patient in a community setting,
- The patient admitted to a hospital, nursing home or other institution.
The pharmacist records an MVE[16].
Medication supply to patients:
- In an ambulatory situation only occurs on the basis of a VV or a repeat VV,
- In a clinical situation occurs on the basis of the MA without the need for a VV.
2.3.8 Process step: Send and/or make available
This step involves information exchange. Information can be sent or made available with different intentions:
- As a request to the prescriber to create a new medication agreement (VMA) or a new dispense request (VVV).
- Informing the prescriber about the processing of the prescription (TA and/or MVE).
- Making available the medication data (TA, MVE), so that fellow health professionals and/or the patient can consult them at a later date.
2.3.9 Postcondition
- A TA may have been created.
- Medication supply may have taken place and, if necessary, the patient has received instructions on how to use the medicinal product.
- Fellow health professionals have been informed or may inform themselves. The prescriber has been informed.
- If necessary, the prescribing physician has been requested to provide a new/modified MA or VV.
2.3.10 Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[17]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
2.3.11 Practical examples
The following specific practical examples have been elaborated:
- New medication agreement, medication dispense of the same product
- New medication agreement, more precise product specification
- Existing administration agreement is adequate
- Proposal to prescriber for medication agreement
- Request and dispense
- Patient requests repeat prescription via physician (reactive repeat)
- Patient requests repeat prescription via pharmacist
- Proactive repeat prescription by pharmacist
- Dispense based on an existing dispense request
- Splitting a prescription
- Starting and continuing a GDS
- Pharmacist changes commercial product
- Adding medication to a GDS
- Discontinuing medication in a GDS
- GDS supplier supplies other commercial product
- Parallel administration agreements with GDS and non-GDS dispense
- Handling a stop-medication agreement
- Dispense with someone else’s administration agreement
- Modification of someone else’s administration agreement
- Deviation from prescribed quantity in DispenseRequest
2.4 Process: administer
This paragraph describes the administration process. This process comprises the compilation of the administration list for (professional) administrators and the administration which is carried out by a (professional) administrator, the patient himself or an informal caregiver. Professional administrators are physicians, nurses and caregivers (home care/institution).
In paragraph 2.4.1, the current situation is described, followed by a description of the new, altered situation.
2.4.1 Current situation
In the current situation, the (professional) administrator, together with the patient, verifies the medication which should be administered, using the available information (paper and/or digital administration list(s), the dosing schedule for, for example, anticoagulant medication, the information on the label), and, subsequently, administers the medication. In case of uncertain administration instructions, the administrator contacts the prescriber or pharmacist. Uncertainties are more likely to occur with an increasing number of prescribers, suppliers and administrators who are involved in the administration process. In the current situation, for administrators, there are several challenges in the transfer of medication data:
- Transfer of medication data between inpatient health care or home care, and hospital health care is missing or is not available in time;
- Medication changes are not received (in time), and medication stops are missing;
- Up-to-date data on the administration list is not available in the case of supply by multiple pharmacies during after-hours care (evening, night or weekend);
- Separate dosing schedules, in addition to the administration list, of (highly) variable dosages, for example anticoagulant medication;
- Problems related to a separate dosing schedule of (highly) variable dosages; for example, loss of the dosing schedule, verbal information regarding changes of the dosing schedule;
- Medication as needed, injection schedules (for example, for insulin) are missing on the administration list (the injection schedules will be worked out in the information standard in the future);
- An overview is missing of all health professionals and healthcare providers which are involved.
In the current situation, the pharmacist compiles the administration list for the (professional) administrator or the patient. This administration list comprises the administration times, which are geared to the rounds of the home care organisation, with account for the pharmaceutical ranges. A paper or digital administration list is used by the (professional) administrators for the registration of the medication administration. A paper administration list (including the administration registration) is in the patient’s home, and is therefore accessible for all professional administrators (irrespective of organisation). The digitally recorded MTDs are only exchanged with other health professionals in exceptional situations (for example, it may be exchanged in the case of admission or special circumstances). With the transition to an electronic administration registration, the administration list (with the recorded administration times) is no longer physically present; instead the administration is recorded in the E-TDR/E-TRS (electronic administration registration/electronic administration registration system) (see paragraph 2.4.8). The involved professional administrators of different organisations (with possibly different E-TRS applications) cannot consult or can only consult with difficulty the registered administration procedures of other organisations, because of registration in different applications and/or databases.
2.4.2 Precondition
The patient administers medication to himself or the patient must be administered medication by a(n) (professional) administrator and, therefore, requires an administration list.
2.4.3 Trigger event
The moment the medicinal products must be administered.
2.4.4 Process step: Administration list
The (professional) administrator or patient receives or consults the medication data needed for the automated generation of the administration list. For the administration list, among other things the medication-building blocks MA, WDS, TA and MTD are needed. In order to compile a complete administration list, the (guide) administration times and dosing instructions are required. The pharmacist and prescriber can enter these data in the TA, and MA or WDS, respectively. Requesting medication data for the purpose of drawing up a administration list is preferably done automatically. Recently stopped medication administrations are also shown in the administration list. In general, pharmacists and prescribers in the ambulatory setting do not register the (guide) administration times and, therefore, they should receive a trigger which indicates that it concerns an ‘administration patient’; a patient who is aided in the medication administration or a patient who requires an administration list. For some medication, it is necessary to provide information on the previous locations of administration; this is registered in InjectionPatchSite in the MTD. In the case of inpatient health care, administration lists are available for all patients, and (guide) administration times are registered by default.
Most (guide) administration times are chosen based on the administration times of other prescribed medication and the rounds of the administrator, especially for MAs and TAs with standard flexible (guide) administration times. Further agreements should be made regarding the (guide) administration times by those concerned in the healthcare field (for example, which deviations from the (guide) administration times are permitted). If the administration of a drug requires a specific time of administration, for example because of medication interactions, this is communicated by entering in the TA and/or MA that the (guide) administration time is not flexible.
The administration list can differentiate between GDS medication (GDS: medication distribution system, in Dutch ‘Geneesmiddel Distributie Systeem’) and non-GDS medication based on the distribution type. This information is extracted from the building block TA. Subsequently, the administrator (administration software) compiles the administration list with differentiation according to administration moment. Prescribers, pharmacists and administrators are responsible for providing the medication data which is necessary for generating the administration list.
2.4.5 Process step: Administering
The following paragraphs describe the different situations in which an MTD is created: Registering a new MTD, correcting a registered MTD and suspending an administration.
2.4.5.1 Registering a new medication administration
The (professional) administrator has received or consulted the medication data that is required for the medication administration. The (professional) administrator, together with the patient, verifies the available medication and the administration data. If agreed and needed, the professional administrator prepares the medication for administration. The medication is administered and the administrator records the administration. Deviations in the medication administration (not administered, adjusted dosage, refusal by the patient, swallowing problems, adverse effects, etc.) are recorded as well, if applicable as a correction or suspension. Corrections and suspensions are explained in more detail in the next paragraphs.
2.4.5.2 Correcting a medication administration
When an MTD is made available but has to be corrected, another MTD is registered. The AdministeredAmount of a correcting MTD can be negative. This makes the sum of the AdministeredAmount from different MTDs the final amount that has been administered. When registering a correction the ReasonForDeviation can be filled in with e.g. ‘Incorrect registration of medication’ or ‘Drug spat out by patiënt’.
2.4.5.3 Suspending a medication administration
When a planned administration cannot be done, it is possible to register that the administration did not take place. This MTD will then have a AdministeredAmount of 0. If the administration is not intended to be given at a later moment, this can be communicated in the Comment data element of this MTD. When the medication is administered at a later moment, this is registered in a new MTD.
2.4.6 Process step: Send and/or make available
In this step, information is exchanged. Information can be sent or be made available with different intentions:
- The recorded MTDs and deviations can be sent for information to a fellow health professional;
- Making available the medication data (MTD), so that fellow health professionals and/or the patient can consult them at a later date. For example, the administration data can be retrieved upon transfer of the patient to another department or institution. Also, a health professional can check which medication is administered to a patient.
2.4.7 Postcondition
The patient has administered medication by himself or has been administered medication. If MTDs have been recorded in an information system, they are sent and/or made available to fellow health professionals and the patient.
2.4.8 Information systems and transaction groups
The prescriber and the pharmacist as well as other administrators and patients (optional) all make use of an information system, respectively, an electronic prescribing system (‘elektronisch voorschrijfsysteem’ - EVS), an electronic client file (‘elektronisch cliëntendossier’ - ECD), a pharmacist information system (‘apothekersinformatiesysteem’ - AIS), a hospital pharmacist information system (‘ziekenhuisapotheekinformatiesysteem’ - ZAIS), an administration registration system (‘toedieningsregistratiesysteem’ - TRS), a thrombosis service information system (‘trombosedienstinformatiesysteem’ - TrIS) and a personal health environment (‘persoonlijke gezondheidsomgeving’ - PGO). These information systems each have different system roles which enable the exchange of data between these information systems as part of the administration process. The information systems may have a function in compiling an administration list, and in the registration, the exchange and the delivering of an MTD. Chapter 6 provides an example of compiling an administration list. The most important elements for the administration process are included in the overview below.
2.4.9 Practical examples
The following practical examples for medication administration have been elaborated:
- Creating an administration list
- Exact administration times required
- Missing (guide) administration times
- Non-GDS medication as needed
- Medication supply by multiple pharmacies
- Change in GDS from the next supply or immediately
- Increasing dosage of GDS in new MBH
- Decreasing dosage of GDS in new MBH
- Change processed by the pharmacist
- Change not processed by the pharmacist
- Extra instructions for administering, explanation in MA, TA and/or WDS
- Medication administration deviates from administration list
- Medication administration without medication agreement and administration agreement
- Medication administration of self-medication
- Correction of an administration
- Medication not administered
- Medication administration on hold
- Medication administration by a prescriber
- Multiple administration organisations
- Feedback to patient through a medication adherence app
- Registration (stop-)MA retroactively with interim MTD
- Registration of separate MTDs per InjectionPatchSite
- Patient transfer to a different department with the same GDS supply date
- Patient transfer to a different department with an earlier GDS supply date
- Patient transfer to a different department with a later GDS supply date
- GDS medication dosage increase, no change in administration times (option A)
- GDS medication dosage increase, no change in administration times (option B)
- GDS medication dosage increase, change in administration times
- GDS medication dosage decrease
- Changing GDS medication to ‘as needed’ medication
- Splitting GDS medication into GDS medication and ‘as needed’ medication
2.5 Process: use
This paragraph describes the process of medication use including registration of medication use by the patient or a health professional. The information recorded by the patient or health professional may be used, among other things, for medication verification by the health professional. During medication verification, medication use is established by the health professional. A picture of this process can be found in Chapter 2.
2.5.1 Current situation
- There is currently a limited number of information systems available in which the patient can record his own use of medication. Exchange to health professionals is also highly dependent on the information system used and is often limited to one specific health professional via, for example, one specific platform/app.
- The current medication profiles are often incomplete and not up to date.
2.5.2 Precondition
The patient has been prescribed medication.
2.5.3 Trigger event
The patient has used the medication or does not use it (anymore).
2.5.4 Process step: Medication use
The patient uses the prescribed medication or self-medication. Medication use may be recorded by
- The patient himself or his informal caregiver,
- A home care or institution nurse and/or
- Another health professional.
This third option often applies in the event of medication verification (see paragraph 2.1). A variety of information systems is available to record medication use: PHR, XIS, EPF [electronic patient file] / ECF [electronic client file], app, etc.
The following can be recorded as medication use:
- Self-medication and other proprietary medication,
- Discrepancies compared to agreements made,
- Confirmation of medication use (promoting compliance),
- Verified medication (see paragraph 2.1),
- Changes as a result of, for example, side effects.
Patients who are being administered medication by a nurse sometimes take the medication by themselves at a later time, depending on the BEM-score (assessment of medication self-management, in Dutch: 'Beoordeling Eigen Beheer Medicatie'); in that case, MTD data may deviate from MGB.
While medication is being used, pharmaceutical care is provided by the pharmacist (see paragraph 2.3.4, e.g. in case of new laboratory values). There may also be follow-up contact during which the pharmaceutical treatment is evaluated (see paragraph 2.2.4).
2.5.4.1 Recording of medication use by the patient
The patient makes use of a medication profile and indicates actual medication use for each medicinal product for which this is relevant. The patient can indicate whether or not he is using the product and/or whether this is ‘medication use according to agreement’ (whereby the MA and/or TA is displayed) or if there were deviations. In case of deviations, it is possible to enter specific deviations (i.e. with regard to the schedule actually followed by the patient), but deviations can also be entered in more general terms (for example, I take the medication: ‘from time to time’, [x] times per [days/week] on average’, ‘1x daily instead of 2x’, ‘not anymore since 22 January yyyy’ or ‘not anymore since about a month’). In case of deviation from the agreements, the patient also indicates the reason for changing or discontinuing.
The patient may also add his own medication to the overview and indicate any side effects as the reason for the changes.
Information about medication use will not always be present. There is also no certainty about the reliability of the information. Sometimes there are agreements between the health professional and the patient about keeping track of medication use, and sometimes the initiative is the patient’s entirely.
2.5.4.2 Recording of medication use by health professionals
During the process of medication verification (paragraph 2.1) or evaluating a pharmaceutical treatment (paragraph 2.2.4) the patient (or his informal caregiver) indicates, for example, that he does not use medication or uses it differently than was agreed. He may also indicate that he uses other medication as well (self-care products or foreign medication). The health professional can record these data as medication use. The data about medication use are recorded in an MGB, with the patient being recorded as the source of the information in Informant, and the health professional as the Author.
Instead of or in addition to recording the MGB, a prescriber may also choose to make a new or modified MA with the patient (in accordance with the process described in paragraph 2.2.5). This may occur when the prescriber sees reasons to adjust the agreement in response to the patient’s actual medication use. When the prescriber sees no reasons to adjust the agreement, he may choose to only record the MGB, perhaps with the remark that he has requested the patient to comply with the existing agreements.
In case of deviations in MGB, a pharmacist may create a VMA for the prescriber (see paragraph 2.3.5) and/or advise the patient to inform the prescriber about the deviations.
The assessment of medication use (effect and side effect) will be recorded by the physician in the medical history or used as a reason to modify or discontinue an MA.
2.5.5 Process step: Send and/or make available
The recorded medication data (MGB) can be sent and/or made available to fellow health professionals and the patient.
2.5.6 Process step: Contacting prescriber by patient
Patients can also contact the prescriber themselves if a new or changed medication agreement or dispense request is required. The process is analogous to the process Contacting prescriber by the pharmacist (paragraph 2.3.5).
2.5.7 Postcondition
The medication list has been updated by the patient and actual medication use has been added. If necessary, the patient has asked the prescriber for a new or modified MA or VV.
2.5.8 Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[18]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process.
Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
2.5.9 Practical examples
The practical examples for medication use are described on the basis of registration by the patient. The prescriber may record medication use in the same way but will rather record the assessment of medication use (effect and side effect) in the medical history or use it as a reason to modify or discontinue an MA.
The following specific practical examples for medication use have been elaborated:
- Self-care product
- Medication from abroad
- Modification at the patient’s initiative
- Discontinuation of medication at the patient’s initiative
- No more supply
- Feedback to patient through a medication adherence app
- Registrations of abnormal medication use by patient due to adverse drug reactions
- Register medication use based on supply
3 Domain-specific handling of the medication process
3.1 After-Hours General Practice clinics (HAP)
The after-hours general practitioner (AHGP) works on behalf of the regular general practitioner (GP). The AHGP may (among other things) start, modify and discontinue MAs in accordance with the process described in paragraph 2.1. If necessary, the AHGP will also create the corresponding VVs. In addition to the generic prescription process, it is possible for the AHGP to use the data element ‘NextPractitioner’. When prescribing new medication the AHGP can use this data element to indicate who the regular GP of the patient is. When stopping or changing medication the AHGP can use this data element to indicate who the original prescriber of the medication was. By doing so the AHGP informs the rest of the involved practitioners whom they may contact when the AHGP is no longer available.
The AHGP will inform the regular GP about the after-hours contact. In principle, the AHGP will follow the generic prescription process, except that a AHGP will carry out certain steps on behalf of the regular GP.
3.2 Mental health care
There are only few planned admissions in mental health care (almost only in departments for eating disorders, clinical rehabilitation, etc.). Most admissions are admissions as a result of acute crisis situations and are therefore comparable to admissions via the emergency departments in hospitals (see also paragraph 4.1.19).
3.2.1 Current situation
In the current situation, patient or family/informal caregivers are asked which medication the patient is using. In most cases, the patient is unable to give an answer (often the reason for admission). Family/informal caregivers (if known) are also not always able to answer this. If this is the case, the admitting physician/psychiatrists will contact the general practitioner or the pharmacist to find out the medication. This is difficult outside office hours and during weekends. When data do come in, only part of them is retyped in the information system of the health professionals.
3.2.2 Process
In the new situation, it will be possible to retrieve the available medication data and medication overviews. On this basis, medication verification, assessment of the pharmaceutical treatment (as soon as possible) and medication administration (in accordance with Chapter 2) can be started. Medication dispense is carried out by public pharmacists on an outpatient basis and, in clinical practice, often by a contracted hospital pharmacy.
As is the case with hospital discharge, the pharmaceutical treatment will be assessed at discharge from a mental health institution (paragraph 2.2.4 and subsequent). Clinical medication is discontinued and, if necessary, new medication is prescribed or temporarily halted outpatient medication is resumed or permanently discontinued. The physician/psychiatrist focuses mainly on psychiatric medication; somatic medication usually remains unaffected.
Letting the psychiatric patient record his own medication use may be experienced as undesirable control instead of it stimulating proper medication use.
The medication data will be made available.
3.3 Nursing, care and home care (VVT)
The medication process in a VVT institution (nursing home, care home and home care) is similar to that of the clinical situation. However, in a VVT institution, a geriatrics specialist is responsible for prescribing medication to admitted clients and medication is being dispensed by one or more community pharmacists, possibly as part of GDS if needed. VVT institutions and home care make use of administration lists. Before administeration of medication, the medication is checked. After administration the actual medicationadministration are signed off. Communication regarding medication administrations between pharmacist, prescriber and nursing staff will be explained in more detail further on in this information standard.
3.4 Hospital admission and discharge
A special situation in the transfer of medication data occurs when a patient is admitted to a hospital. At the moment of admission and of discharge, a lot of changes can take place in the MAs and TAs. That is why this subject is separately illustrated in this chapter. This process is described in more detail in instruction manuals for caregivers and software suppliers.
3.4.1 Prior to admission
Before a patient is admitted to a hospital, changes in medication may be necessary. The prescriber during admission and patient can agree upon starting new medication or stopping continuous medication a few days before admission. At the time of this agreement, the admission date is often not yet known. In such a case, the prescriber during admission registers an MA or stop-MA and mentions in the data element Condition (in PeriodOfUse) how many days before admission the patient has to start or stop the medication. If Condition is filled, it should be clear to caregivers that the PeriodOfUse is uncertain.
3.4.2 During admission and at time of discharge
When a patient is admitted, the following situations can occur, concerning the outpatient medication:
- Unchanged continuous use,
- Change in dosage,
- Generic substitution (active ingredient remains the same),
- Pharmacological substitution (active ingredient changes, but medication is registered for the same indication),
- (Temporary) stop.
Below, for each situation, an explanation is given for registration of the medication of the patient.
3.4.2.1 Unchanged continuous use
The patient continues using the outpatient medication during admission.
Both MA and TA continue during admission and after discharge.
3.4.2.2 Change in dosage
The patient uses the outpatient medication during admission, but in a different dosage.
The MA for outpatient medication is stopped at admission. During admission, a new MA is registered with the changed dosage. At discharge, the prescriber decides which dosage will apply for the outpatient medication and registers a new MA. When registering the MA the prescriber can make use of the data element ‘NextPractitioner'. With this data element the prescriber can indicate who needs to take on the authorship of the MA after discharge. The TA is also stopped at admission. During admission, a new TA is registered, based on the new MA. At discharge a new TA will be registered if a new MA is registered.
3.4.2.3 Generic substitution
The patient uses a different medicinal product during admission, but with the same active ingredient and dosage as in the outpatient medication.
The MA for outpatient medication continues during admission and after discharge. The TA for outpatient medication is stopped at admission and continued at discharge. During admission, a new TA is registered. This TA will be stopped at discharge.
3.4.2.4 Pharmacological substitution
The patient uses a different medicinal product during admission, with a different active ingredient from the outpatient medication. This medicinal product, however, is registered for the same indication as the outpatient medication.
The MA for outpatient medication is stopped at admission and will be continued again at discharge. When registering the MA for after discharge, the prescriber during admission can use the data element ‘NextPractitioner’. With this data element the prescriber can indicate who needs to take on the authorship of the MA after discharge. During admission, a new MA is registered. This MA will be stopped at discharge. The TA for outpatient medication is also stopped at admission and resumed at discharge by making a new TA with the same content. During admission, a new TA is registered. This TA will be stopped at discharge.
3.4.2.5 (Temporary) stop
The patient (temporarily) stops using the outpatient medication during admission.
The MA for outpatient medication is (temporarily) stopped at admission. If the medication is stopped temporarily, a new MA will be registered at discharge. When registering the MA for after discharge, the prescriber during admission can use the data element ‘NextPractitioner’. With this data element the prescriber can indicate who needs to take on the authorship of the MA after discharge. If the medication is stopped definitively, the MA will not be resumed. The TA for outpatient medication is also (temporarily) stopped at admission. If the medication is stopped temporarily, this TA will be resumed at discharge by registering a new TA with the same content. If the medication is stopped definitively, there will be no new TA after discharge.
4 Description of practical examples
This chapter contains a description of various practical examples. Specific practical situations have been described for the various subprocesses. A large number of the practical situations are based on general medical practice, but are illustrative of similar situations in a different setting. This chapter assumes the knowledge as previously described in Chapter 2. Some of these practical examples are accompanied by a visualisation. In these visualisations the acting stakeholders are shown, as well as the building blocks described in the situation. Changes in building blocks and new building blocks are shown as they occur in time.
| Legend visualisations | ||||
|---|---|---|---|---|
| characters | ||||
| Prescriber
Recognizable by their stethoscope |
Pharmacist
Recognizable by their pestle and mortar |
Patient
Recognizable by their bandage |
Administrator
Recognizable by their syringe |
Healthcare provider
Recognizable by their hat |
| Symbols | ||||
| Start of a relevant building block
The color indicates the type of building block |
Start of a stopped building block
Stopped building blocks are grey |
End date of a building block
The color indicates the type of building block |
End date of a stop-building block
The color of the outline indicates the type of building block |
A building block without an end date
The color indicates the type of building block |
| An instance of VV or MVE
The color indicates the type of building block |
Labtest value | Direction of sent message | Signal from the information system | Textbox for clarification |
4.1 Practical examples, Prescribe
4.1.1 Short-term medication
A 35-year-old female patient with a history of urinary tract infection brings her urine to the doctor’s assistant and tells her she has the same symptoms as the previous times. The assistant enquires about other symptoms such as fever and pain in the flank and checks the urine. There are no other symptoms and the urine reveals a urinary tract infection. She tells the patient that the general practitioner will prescribe a course of treatment and that she can pick it up from the pharmacist later. The general practitioner checks the previous courses of treatment and any cultures and records an MA. On 27 January it was agreed:
- Nitrofurantoin CR capsule, 100 mg, 2x daily, 1 capsule, from now on[19] for 5 days.
He records this MA in his information system. The general practitioner then immediately also makes a VV for the pharmacist chosen by the patient:
- Nitrofurantoin CR capsule, 100 mg; 10 capsules.
The general practitioner also records this VV in his information system.
The general practitioner consults with the patient to find out from which pharmacist she wants to pick up the medication. The prescription (MA and VV) is sent to the pharmacist and the medication data are made available to fellow health professionals (MA only) and patient (MA and VV).
4.1.2 Continuing medication
The process for continuing medication is practically the same as for short-term medication (see paragraph 4.1.1), except that in the case of continuing medication the MA is made for an indefinite period of time.
For example: the prescriber agrees with a patient who has hypertension that he should use a diuretic on a continuous basis. On 30 March yyyy it was agreed:
- Hydrochlorothiazide tablet, 12.5 mg; 1x daily, 1 tablet; from now on for an indefinite period.
For example, the prescriber chooses in the VV an initial supply quantity of 100 tablets.
4.1.3 Hard endDateTime for PeriodOfUse
A startDateTime, endDateTime and/or Duration can be included in the PeriodOfUse of an MA. If a hard endDateTime is desired, this must also be explicitly stated in the explanation. Only including an endDateTime is not sufficient because this does not provide enough clarity about the intention of the prescriber.
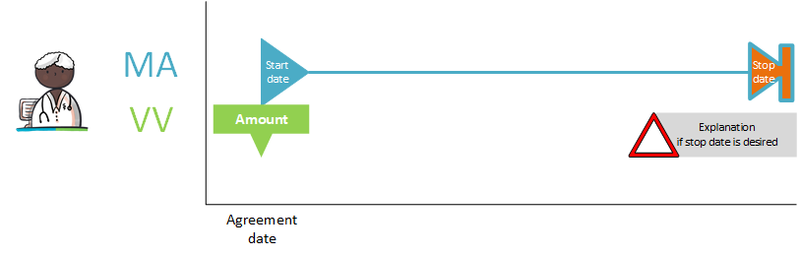
4.1.4 Medication as needed
A 31-year-old female patient has had headaches a few times in a year, with migraine now being diagnosed. The general practitioner prescribes:
- Rizatriptan tablets, 10 mg, as needed 1d1t under the tongue. First tablet at the next clear migraine attack.
The general practitioner records the MA and makes a VV:
- 6 sublingual rizatriptan tablets, 10 mg.
4.1.5 Course of treatment as needed starting in future
A 60-year-old patient with post-thrombotic syndrome experiences erysipelas (severe infection of the leg which can require admission if medication is not started quickly) sometimes every other year, sometimes three times a year. At the request of the patient, the general practitioner prescribes an antibiotic treatment that the patient can start immediately in case of a recurrence of erysipelas. The following MA is created:
- Flucloxacillin capsules, 500 mg, 1 capsule 4x daily, for 10 days. In the MA is indicated (condition): start in case of recurrence, as needed.
The general practitioner immediately creates a VV:
- Flucloxacillin 500 mg, 40 capsules.
To prevent the MA from being relevant to the medication overview indefinitely, a stop-MA is created with the actual startDateTime or endDateTime as soon as this is known to the prescriber.

4.1.6 Two dosages of the same medication at the same time
A 79-year-old man with metastatic prostate cancer is increasingly suffering from pain and his medication is being changed. The specialist prescribes oxycodone tablets, 10 mg, 1 tablet 4x daily and as needed for pain during the night, 1 tablet 1x daily, on October 9. The specialist records this in one single MA with two dosing instructions (in data element InstructionsForUse/DosingInstructions) and creates a VV for 60 tablets of oxycodone of 10 mg.
4.1.7 The same medicinal product with different strengths at the same time
The same 79-year-old patient (see paragraph 4.1.6) is experiencing even more pain. The specialist discontinues the 10 mg tablets of oxycodone on October 16 and prescribes 20 mg retard tablets of oxycodone, 2 tablets 3x daily, and 20 mg normal release tablets of oxycodone as needed in case of nocturnal pain, 1 tablet 1x daily. The specialist records this in two MAs (with, for the time being, two separate MBHs) and creates a VV for both MAs, respectively 45 retard tablets of 20 mg oxycodone and 30 tablets of 20 mg oxycodone (normal release).
4.1.8 Extra particularities in the medication agreement
When selecting a medicinal product, it is possible to deviate from what is expected. This can be clarified in the data elements ‘Comment’ or ‘MedicationAgreementAdditionalInformation’ of the MA. The data element ‘MedicationAgreementAdditionalInformation’ contains a list of particularities of the MA that are relevant for pharmacovigilance and fulfilment by the pharmacist. The element ‘Comment’ is used for particularities that cannot be registered in a coded manner in ‘MedicationAgreementAdditionalInformation’. Below are three examples of the use of these elements.
Example 1: the hospital uses a different formulary than a community pharmacy
For reasons of efficiency the hospital has opted for a single gastric acid inhibitor: pantoprazole. At admission, a patient with omeprazole is switched to pantoprazole for the duration of the hospitalisation. At discharge, the patient returns to omeprazole. Without intervention, it is possible that the patient will use omeprazole and pantoprazole instead of just the omeprazole.
In the MA of the hospital for pantoprazole, a remark can be made in the data element ‘Comment’. This makes it clear that pantoprazole is the replacement of omeprazole.
Example 2: Half-strengths
The hospital sometimes has tablets available with half the strength of the normal commercial preparation. When a patient enters the hospital with a prescription of 25 mg of chlorthalidone, once a day half a tablet, he will receive 12.5 mg of chlorthalidone once a day while in the hospital. This means nursing staff does not have to break tablets.
There is a risk here that the patient will use 25 mg again at home, but now a whole tablet at the time. In the MA of the last chlorthalidone 25 mg the element ‘MedicationAgreementAdditionalInformation’ can be used to indicate whether this was a deliberate increase.
Example 3: Specific commercial product (HPK), medical necessity
When a prescribed product has to be a very specific commercial product (HPK), the prescriber can specify this in the MA. The prescriber indicates uses the element ‘MedicationAgreementAdditionalInformation’ of the MA with the code ‘medical necessity’ to communicate that a specific commercial product is necessary.
4.1.9 New medication agreement, no dispense request
First example
A 50-year-old man comes to the general practitioner with back problems. The symptoms have been present for three weeks and he is already using paracetamol. The general practitioner agrees with the patient that he can additionally use diclofenac:
On 30 January it was agreed:
- Diclofenac tablet, 50 mg, 1 tablet 3x daily, from now on for 3 weeks.
He records this MA in his information system. The patient indicates there is sufficient supply at home: his wife still has an ample supply of diclofenac left because of a different problem a year ago.
A VV is therefore not needed and the pharmacist does not supply any medication.
The general practitioner makes the new MA available to fellow health professionals and the patient.
Second example
Mr Simons receives medication from the pharmacy weekly. In the past there were many requests for extra medication, which led to clear agreements about the dispense policy.
This is about:
- MA: Diazepam, 5 mg, 1 tablet 4x daily, from 1 January yyyy for an indefinite period
- VV: 28 tablets with 10 repeats; remark: weekly medicatieverstrekking
The VV is repeated every 11 weeks. The last VV is planned for 3-6-yyyy:
- one week (28 tablets) with 10 repeats
The last few years have been quiet. Mr. Simons is no longer asking for extra medication. At the last consultation (at 31-3-yyyy) he indicated that since three weeks diazepam, 3x daily, is sufficient. This has been recorded in a MA:
- 1 April yyyy Diazepam, 5 mg, 1 tablet 3x daily, from 1 April yyyy for an indefinite period.
The previous MA will be terminated as of 31 March yyyy (see discontinuation of medication in paragraph 2.2.5.3).
In the current situation, the general practitioner would have called the pharmacy to make sure that 21 tablets of diazepam would be given with the following medicatieverstrekking, instead of 28. No new VV would have been needed at the time.
In the new situation, the general practitioner sends the new MA to the pharmacy. Based on the new MA, the pharmacy supplies 21 tablets per week. With the new MA a new VV is not instantly required. The previous VV is still sufficient.
4.1.10 New dispense request under existing medication agreement
A prescriber may also create a new VV as part of an existing MA. This MA may have been created by a different prescriber, for example a psychiatrist. This concerns repeat medication. The practical example for repeat prescription is described in paragraph 4.2.6.
4.1.11 Dosage change (sufficient supply)
Patient (37 years old, asthma) visited the pulmonologist on 13 August yyyy for a checkup of his asthma. The pulmonologist has established that patient’s asthma is not adequately controlled.
The patient is currently using beclomethasone in accordance with a previously made and registered MA: on 10 June yyyy it was agreed:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; 1 inhalation 2x daily; from 10 June yyyy for an indefinite period.
On 13 August yyyy, the pulmonologist agrees with the patient on a dosage increase:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; 2 inhalations 2x daily; from 13 August yyyy for an indefinite period; reason for adjustment: insufficient effect.
The previous MA of 10 June yyyy is no longer valid: it is terminated (see Changing medication in paragraph 2.2.5.5). The patient still has a sufficient supply, a VV is therefore not needed.
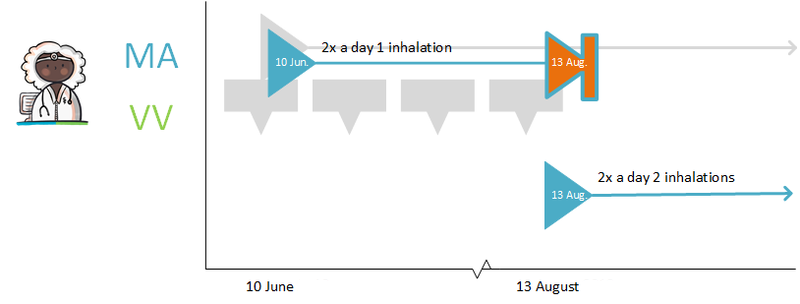
In case the patient has no more supply, a new VV will be created.
The information system of the pulmonologist makes the new MA available to the other health professionals of this patient. This modification is received by the pharmacist (fellow health professional). The pharmacist processes the modification. The pharmacist may also choose to accept the modifications automatically.
4.1.12 Prescription no longer needed after first dispense request
A 16-year-old woman has a boyfriend and does not want to become pregnant. After explanation she opts for the pill. The general practitioner prescribes ethinylestradiol/levonorgestrel tablets of 20/100 µg, 1 tablet 1x daily for 21 days, then no tablet for 7 days, then another 21 days of taking a tablet once a day. Start on the first day of the next menstruation. The general practitioner records the MA and creates a VV for 63 coated microgynon 20 tablets. He explains to her that if she does not experience any problems, she can continue to get the pill via the pharmacy.
Three months later, the woman sends a request for repeat supply to the pharmacy. The pharmacy supplies the product and communicates the MVE to the general practitioner.
4.1.13 Discontinuing medication
Patient (37 years old, asthma) visits the pulmonologist on 13 August yyyy for a checkup of his asthma. The patient is suffering from a side effect. The patient is currently using Beclomethasone in accordance with a previously made and registered MA. On 10 June yyyy it was agreed:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; 1 inhalation 2x daily; from 10 June yyyy for an indefinite period.
On 13 August yyyy, the pulmonologist agrees with the patient to discontinue the medication (medicamenteuze behandeling is discontinued). Medication verification is also applicable here. In addition, medication monitoring may also be relevant here, for example if gastroprotective drugs are being used because of the use of the medication to be discontinued (the information system will not always signal this automatically).
The pulmonologist records:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; stop type ‘discontinued’; from 13 August yyyy. Because of a side effect.
The stop-MA is sent to the pharmacist and made available to fellow health professionals and the patient. In addition, the pulmonologist decides to send the stop-MA to the general practitioner as well.
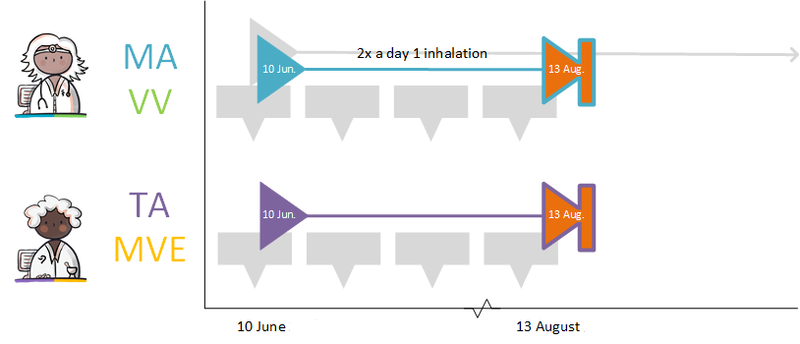
4.1.14 Temporarily halting/resuming medication
A patient is being treated with simvastatin (cholesterol-lowering agent): 40 mg, 1 tablet 1x daily, for an indefinite period. Because of a throat infection in combination with an allergy for the first antibiotic of choice, Clarithromycin, 250 mg, 1 tablet 2x daily (antibiotic) is prescribed for 1 week. During that week, simvastatin is temporarily halted because of an interaction.
The general practitioner records:
- Simvastatin 40 mg; halt temporarily; for 1 week; reason: interaction
- Clarithromycin, 250 mg; 1 tablet 2x daily; for 1 week (and a corresponding VV)
The general practitioner sends a stop-MA with stop type 'suspended' to the pharmacist to temporarily halt the simvastatin. He also makes and sends a new MA to resume the simvastatin after a week and an MA with VV for the clarithromycin. See also Temporarily halting and resuming medication in paragraph 2.2.5.4.
4.1.15 Temporarily halting for an intervention
A 62-year-old man uses acetylsalicylic acid, 100 mg, 1 tablet 1x daily because of coronary disease. He has an intestinal polyp that must be removed. The general practitioner informs him that he should stop using acetylsalicylic acid three days before the intervention and resume the day after the intervention. The general practitioner records the temporary halt in an MA with an endDateTime 3 days before the intervention; stop type ‘suspended’; reason for halting: ‘intervention’. In the (second) MA for resuming, 1 day after the intervention is registered as the startDateTime. The general practitioner sends the MAs to the pharmacist and the gastroenterologist.
When the date of the intervention is unknown or uncertain, the explanation will indicate that the medication should be stopped three days before the intervention and resumed one day later. The duration of the temporary halt is then 4 days. See also Temporarily halting and resuming medication in paragraph 2.2.5.4 and Prior to admission in paragraph 3.4.1.
4.1.16 Paper prescription - no more allowed
The Dutch law Wegiz (Wet elektronische gegevensuitwisseling in de zorg) states that per January 1st, 2024, prescriptions on paper only are not allowed anymore. Therefore the contents of this practical example have been removed.
4.1.17 Carrying out medication verification and evaluation of foreign or self-medication
When medication cannot be found in the information system (such as foreign medication and self-medication), this medication will be recorded as MGB (see paragraph 2.2.5).
4.1.18 Day treatment
An admission for day treatment is comparable with an outpatient consultation or an emergency admission during which the medication is supplied by the hospital pharmacist. In the case of admission for a day, extensive medication verification does not usually occur. In the case of admission for a day, the medication prescribed is recorded (often on the basis of protocols according to which verification is carried out afterwards) and administered.
For treatments with a course of cytostatics, for example, there is an MA, but it is not always clear in advance when the medication dispense/administration takes place. Reason: treatment courses are often postponed and then supplied and administered later.
4.1.19 Starting with medication before admission
Prior to cataract surgery, Nevanac is started three days before the surgery. The eye drops are used until three weeks after surgery (a total duration of medication use of 24 days). The specialist creates an MA for Nevanac, 1 drop in the morning, for 24 days. When the date of the surgery is unknown or uncertain, mention can be made in PeriodOfUse/Condition that Nevanac should be started 3 days before surgery takes place. See Prior to admission in paragraph 3.4.1.
4.1.20 Emergency admission
In the event of an emergency admission, extensive medication verification is not carried out beforehand as would normally be the case with clinical admissions. Agreeing on medication with the patient is also often not possible in the event of an emergency admission. Often, the patient is administered medication as soon as possible and verification only takes place afterward
4.1.21 Interim discharge
When a patient temporarily leaves the hospital/institution to go home, for example, for weekend leave, the clinical medication continues. In order to keep the patient’s own general practitioner informed, the medication overview is provided to the patient and the medication data are made available.
4.1.22 Transfer to another institution
In case of a transfer to another institution, the MBH is evaluated (see paragraph 2.2.4 and paragraph 2.2.5). The medication data are sent to fellow health professionals and the patient and/or made available to them. The new attending physician evaluates the MBH and determines the institutional medication.
4.1.23 Do not dispense before
The prescribing physician creates a VV on 14 April yyyy but wants to indicate in that same VV that medication dispense may only take place on or from a later date, for example, 23 April yyyy. This cannot be recorded in a structured manner in the VV, but is included in the Comment data element (as free text).
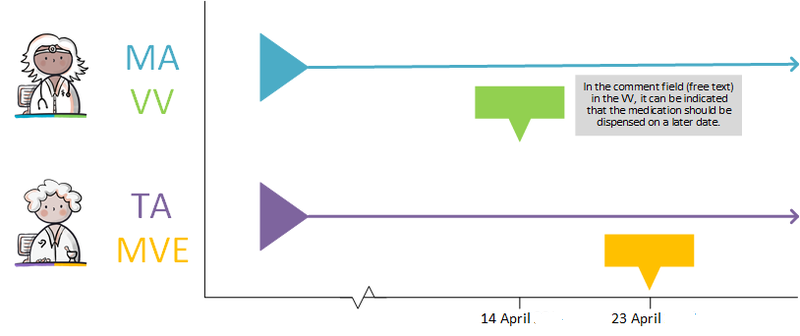
4.1.24 Discontinuation of medication by third parties
Medication can be discontinued by the prescriber himself (see paragraph 2.2.5.3 and example in paragraph 4.1.13) but also by another prescriber. For example, the specialist can end MAs made by a general practitioner that are up to date according to the information system, but turn out not to be, for example after medication verification. When a health professional discontinues medication, he creates a new stop-MA. The health professional cannot modify someone else's MA, only create a stop-MA. He sends the stop-MA to the health professional who made the original MA to inform him of this. The health professional of the original MA then processes if possible the stop-MA in their own information system.
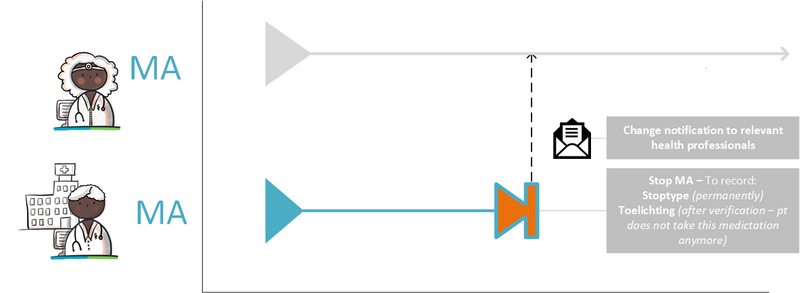
4.1.25 Two PRKs in a single pharmaceutical treatment
In certain circumstances, the pharmacy is allowed to select a different PRK for the TA than is indicated in the MA made by a prescriber. If, for instance, a failure of the prescriber’s information system would result in only the communication of the TA, a subsequent prescriber will only have this TA with the new PRK. The next prescriber will then probably assume that this PRK is also the PRK of the MA. A modification of the MA will then also result in a stop-MA (without referring to the original MA) and a new MA on the basis of this PRK. This means that there are now two MAs with different PRKs under the same MBH. These two MAs continue to exist. After any information system failure, (the information system of) the prescriber must check whether there have been changes and implement them, if necessary (see also paragraph 4.1.24).
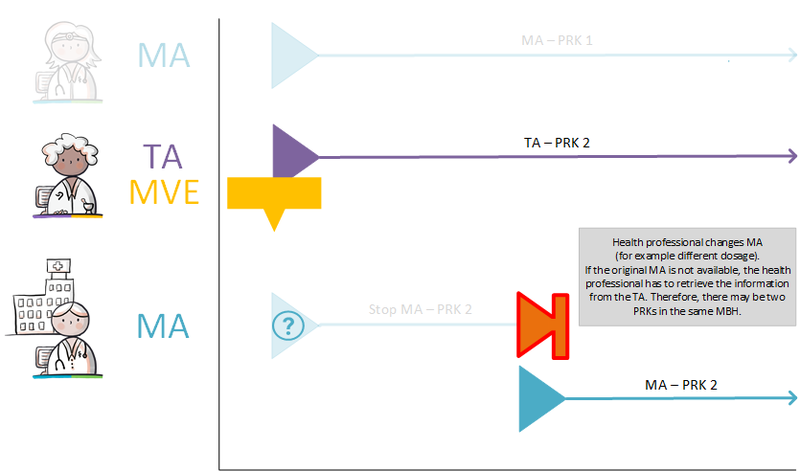
4.1.26 Creating a medication agreement after the fact
In emergency situations, for example, it may happen that the MA is only created after the medication has been supplied or administered. This may lead to conflicting MAs. This means that one or more MAs must be discontinued in order to prevent the occurrence of parallel MAs. The only exception for parallel MAs is described in paragraph 1.3.3.
4.1.27 Single medication use
For medications intended for single use, the period of use can be specified with a startDateTime and Duration, a start and endDateTime, or a Duration and endDateTime. An example of this is a situation where the patient is required to take diazepam once, the day before surgery. The prescriber then indicates the period during which this use should occur.
4.1.28 Provisional and final medication order
The prescriber at the clinic prescribes medication. This is recorded in a provisional medication order (the MA). The hospital pharmacist verifies this order and records it as a final medication order (the TA). See also [3].
4.1.29 Inadvertently ‘outstanding’ medication or 'orphans'
In a transitional situation and in a situation where not every XIS is connected, MAs for the same treatment may exist that have been created by different information systems under different MBHs. These MAs should ideally be combined in a single MBH. This particularly applies to medication with an open endDateTime, as this medication may have been discontinued under a different MBH. The MA therefore remains open, resulting in continued medication dispense. This is a so-called ‘orphan’ (a building block that has been registered, but in which, at a certain point, the health professional no longer has an active role. However, his information system continues to provide the building block, even if it has already been discontinued elsewhere). For medication with an endDateTime, this problem will solve itself over time. Therefore, it is not necessary to take any action. When medication verification or medication assessment reveals that the medication is inappropriately still ‘open’, a stop-MA is created under the same MBH. This stop-MA is sent to the the original prescriber. The original prescriber processes the stop-MA into their own information system. See also paragraph 4.1.24 and paragraph 4.1.37.
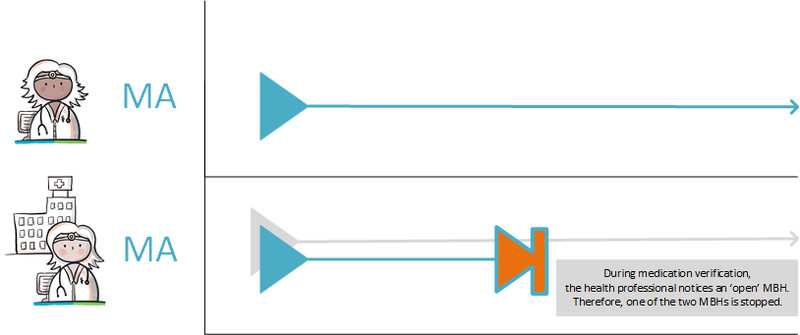
4.1.30 Missing digital medication agreement at admission
If no digital MA is available when medication verification is carried out, a new MBH is started by recording MGB. When this MGB has to be discontinued, a stop-MA is created under the same MBH that refers to the recorded MGB. If possible, the stop-MA is sent to the original prescriber and the patient's pharmacy.

4.1.31 Own articles (90 million numbers)
It is possible to exchange a 90 million number. The condition is that within an organisation a 90 million number once created may never be modified. During the exchange, the root OID (unique technical identification of the organisation) in combination with the 90 million number ensures that it is unique compared to all 90 million numbers form other organisations. The substances that make up this article are included as ingredients in the MA, in data element AgreedMedicine. At least one ingredient must be registered, as is the case with magistrals.
4.1.32 Free-text prescribing
It is, in exceptional circumstances, possible to prescribe without a code from the G-Standard. This is what we understand as "free-text prescribing". This is only permitted when there is no suitable code available in the G-Standard, for example, with investigational medication. Free-text prescribing is thus explicitly the exception, among other reasons because medication monitoring cannot be performed without a G-standard code.
4.1.33 Dosing with minimum interval
Medication can be prescribed at a fixed interval (for example: 2 tables every 6 hours). Prescribing medication with a minimum interval should be partly in free text. For example, if needed, a maximum of 3 times a day, a minimum of 6 hours between the intake moments: if needed, a maximum of 3 times a day is recorded in the standard way, in InstructionsForUse. The instruction “at least 6 hours between intake times” is recorded in free text in AdditionalInstructions.
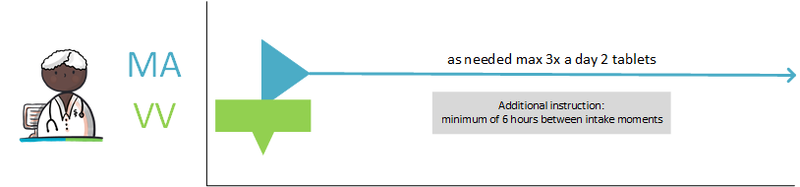
4.1.34 Dispense request with number of repetitions
The prescriber makes a VV with an MA in which she enters the NumberOfRefills. The prescriber hereby indicates to the provider that he may make additional dispenses.
The number of refills in combination with the quantity to be supplied results in: (NumberOfRefills + 1) X Amount. In case of “NumberOfRefills = 3” and “Amount = 30 units” the provider may supply 4 times 30 units (total 120 units).
The number of refills in combination with the duration of medication usage results in: (NumberOfRefills + 1) x PeriodOfUse/Duration. The amount to be supplied depends on the dosage. In case of “Dosage = 3 x per day 2 units”, and “PeriodOfUse/Duration = 30 days” the provider may supply 4 x (6 units per day X 30 days = 180 units) = a total of 720 units.
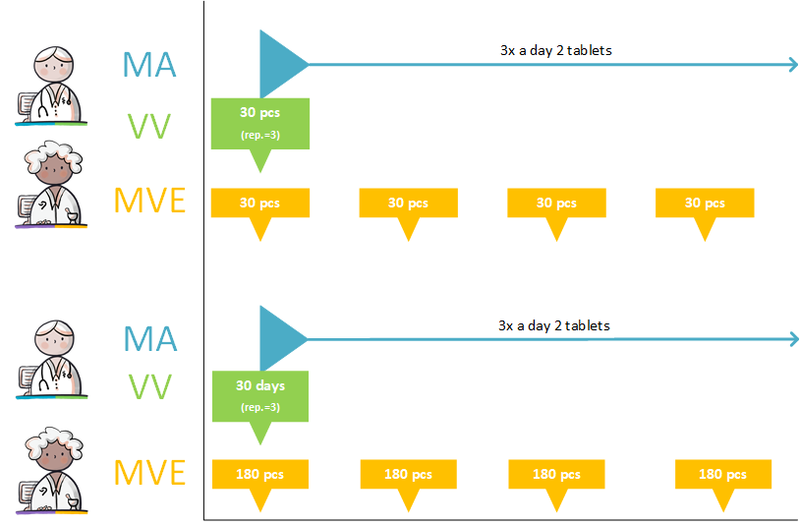
4.1.35 Prescribing non-medicines
Non-medicines can be prescribed from the G-standard at HPK level. For example, this could be an inhaler (Aerochamber, HPK 1915185) as an aid to prescribed aerosols. Non-medicinal products are not applicable for the medication overview or medication monitoring.
4.1.36 Send renal function value in the prescription
A) Renal function value with a new prescription:
For the prevention of stroke, a patient (male, 65 years old) is prescribed Dabigatran for the first time. The patient has renal impairment and the prescriber has a recent renal function value available. Based on this renal function value, the prescriber deviates from the standard dose and makes an MA with a lower dosage of Dabigatran twice a day 1 capsule of 110 mg, starting from January 15, yyyy. The prescriber also makes a VV and sends the prescription (MA, VV and the laboratory result of the renal function value) to the pharmacist.
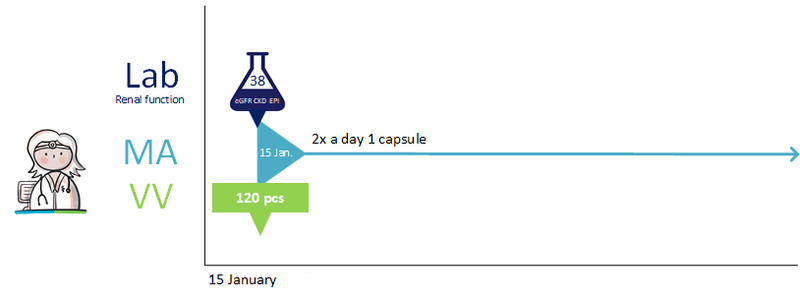
B) Renal function value reason for a change:
A 70-year-old man was prescribed 1 tablet of metformin 500 mg 3 times a day a few days ago (MA and VV). At the same time, the doctor initiated a blood test to determine the renal function value of the patient. The value was not yet known at the time of prescription.
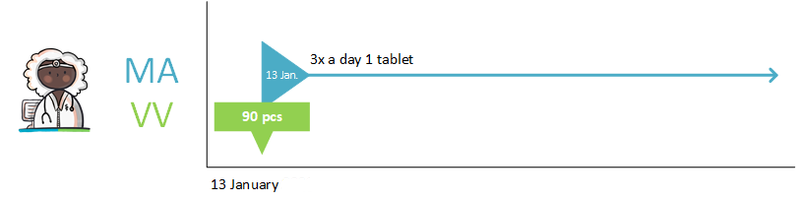
The blood test shows that renal function is impaired and gives reason to adjust the MA. The prescriber adjusts the dose on January 17, yyyy to 2 tablets metformin 500 mg twice a day and discusses this with the patient. The new prescription (the 'technical' stop-MA, new MA and the laboratory result of the renal function value) is sent to the pharmacy by the prescriber. Because the patient had already collected the medication and therefore has enough stock, no new VV is sent along.
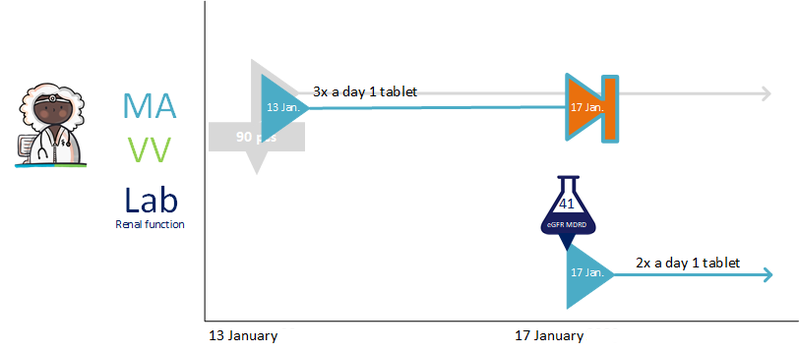
C) Renal function value due to drug:
The patient is prescribed Nitrofurantoin 100 mg, 1 capsule twice daily as a 5-day course due to a bladder infection and must start immediately on January 6, yyyy. With this drug, the renal function value (if known and not older than 13 months) needs to be sent along. The prescriber has a renal function value available for this patient, but this does not lead to an adjusted dosage. The prescriber sends the prescription (MA, VV and the laboratory result of the renal function value) to the pharmacist.
4.1.37 Cancelling a prescription that was sent earlier
A patient visits the general practitioner (GP) one morning because of stomach complaints. The GP prescribes pantoprazole and sends the prescription (MA + VV1) to the preferred pharmacy as known to him. In the afternoon the patient calls the GP. He hasn’t picked up the prescription yet and he would like to pick it up at another pharmacy due to a recent move, but forgot to ask this morning. The GP resends the prescription to the old pharmacy, but the VV in the newly sent prescription contains a CanceledIndicator (MA + VV1 “cancelled”), so the pharmacist knows he should not dispense on this prescription. The prescriber then sends a new prescription (MA + VV2) to the patient's new pharmacy. The patient can pick up the medication there.
In medication building blocks it looks as follows:
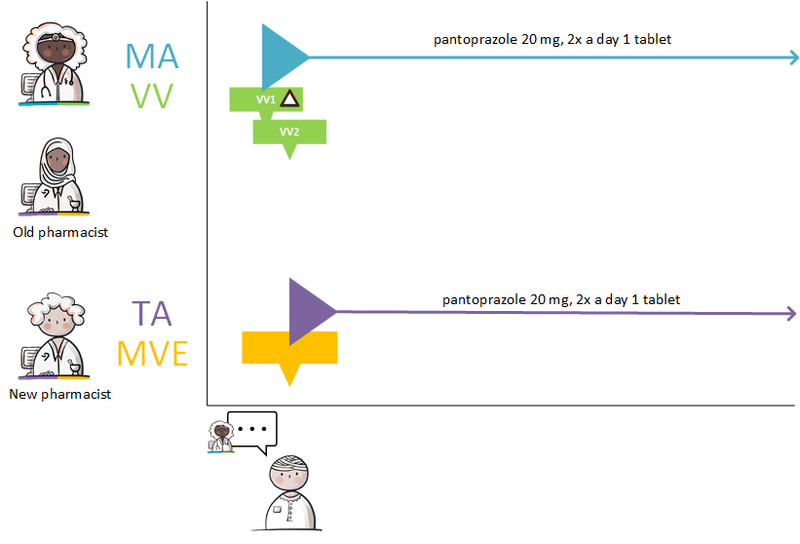
The transactions look as follows:
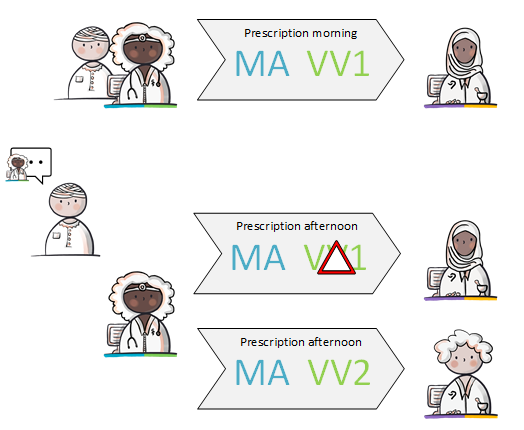
4.1.38 Modification of someone else's medication agreement
A patient takes 40 mg Lisinopril once a day because of hypertension. This MA was previously made by the GP with the patient. The patient is admitted to the A&E department because of a fall due to dizziness. The specialist notices hypotension and decides to reduce the dose of Lisinopril to 20 mg once a day. The specialist then stops the current MA and starts the new MA with a lower dose. With this he changes the MA with the patient. The specialist sends a stop-MA and the new MA to the original prescriber (the general practitioner). The prescriber of the original MA processes the stop-MA in their own information system.

4.1.39 Setting up a variable dosing regimen
The prescriber prescribes anticoagulants and states in the MA that the medication is used as recorded by the schedule of the thrombosis service (‘gebruik volgens schema trombosedienst'). The prescriber sends a VV to the pharmacist and the patient is registered with the thrombosis service. In order to bridge the period until thrombosis care can take over, the prescriber creates a WDS for the first period. After registration, the thrombosis service creates a dosing regimen that overwrites or succeeds the previous regimen. From this moment on, the thrombosis service takes over creating the dosing regimen from the original prescriber. As the WDS is a separate medication building block, the medication overview remains unchanged (the MA is specified, not altered by the WDS). The WDS is used to compile the administration list, together with the medication data in the MA, TA and MTD.
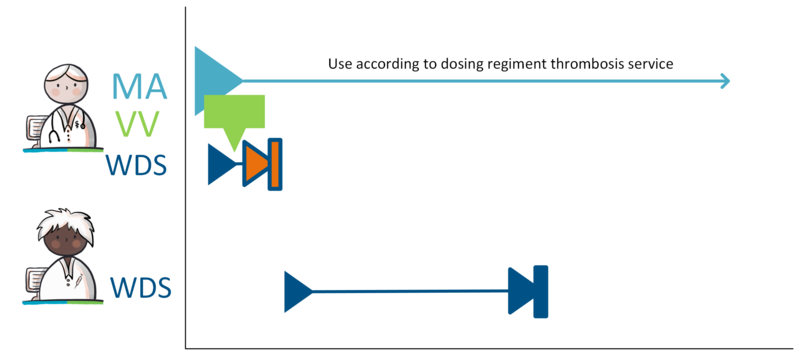
4.1.40 Changing a variable dosing regimen during period of use
It could be necessary to revise the dosing regimen from a WDS before the scheduled endDateTime. For example, when the patient unexpectedly has to undergo minor surgery. In this case, the prescriber stops the current WDS with a technical stop (not visible to the user) and creates a new WDS with a few 0 doses the days before the procedure. In the meantime, the MA continues as usual.
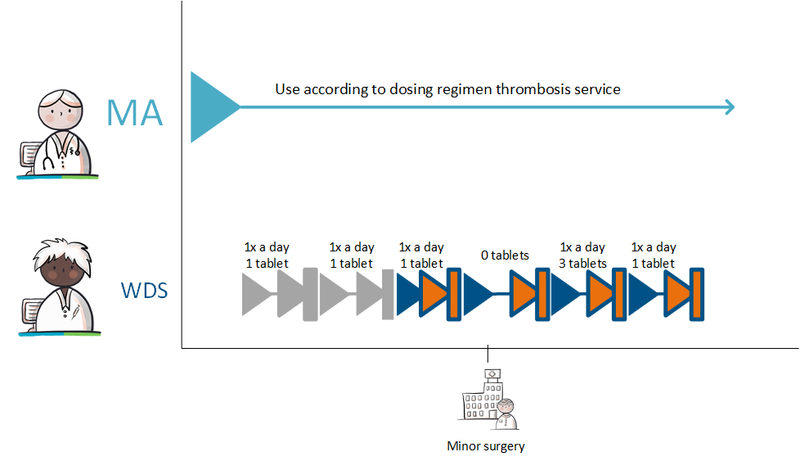
4.1.41 Stopping medication with a variable dosing regimen
When the patient has to (temporarily) stop using anticoagulants completely, this is recorded at the level of the MA. The thrombosis physician creates a stop-MA. This also stops the underlying WDS and the associated TA. The original prescriber also processes the endDateTime in his MA as described in 4.1.29 Inadvertently outstanding medication or 'orphans'.
If the anticoagulants need to be restarted after several months, the original prescriber of the anticoagulant medication does this by following the process of starting a WDS, i.e. by creating a new MA and a first WDS (see the practical example Setting up a variable dosing regimen).
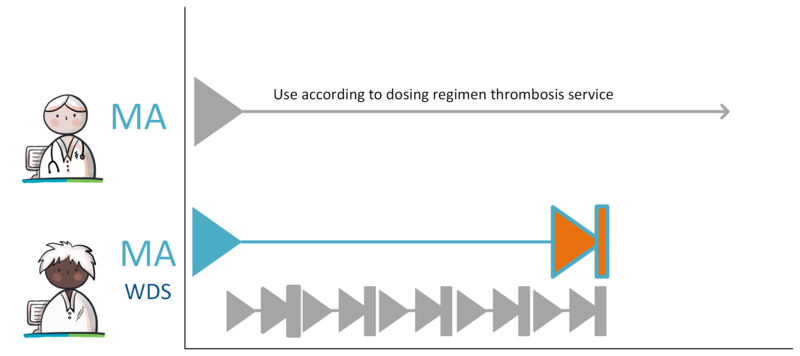
4.1.42 Merging building blocks under one MBH
A patient has an active MA from their general practitioner. The patient has not given permission to make their medication information available. When a nurse wants to register the patient's medication use, the nurse will not be able to register this under the same MBH the MA is active in. When the patient later does give permission for making their medication information available, multiple building blocks become available under different MBHs that should be in one MBH. Merging the MBH works according to the implementation guide for migration and hybrid situations.
4.1.43 Changing someone else's MA by non-prescriber with delegated responsibility
The medical specialist prescribes 300 mg of Niraparib once a day to treat cancer. 24 hours before the scheduled first administration, the hospital pharmacist reviews the lab values and decides that the dosage needs to be adjusted to 200 mg once a day.
There is an agreement within the hospital that the pharmacist has delegated prescribing authority (see paragraph 2.2). The pharmacist modifies the medical specialist's MA. The medical specialist remains author of the MA, as the pharmacist acts on his/her instructions.
4.2 Practical examples, Dispense
4.2.1 New medication agreement, medication dispense of the same product
The patient is at the pharmacist on 27 January yyyy to collect his medication. The pharmacist opens the file of the patient and verifies the medication file. It is a new MA for this patient. The relevant information of the prescribing physician is displayed on the screen of the pharmacist.
MA: Nitrofurantoin CR capsule, 100 mg, 1 capsule 2x daily, from now on for 5 days.
VV: Nitrofurantoin CR capsule, 100 mg; 10 capsules.
The pharmacist selects a product on the basis of the MA and other factors (such as the pharmacist’s stock and the preference policy of the healthcare insurer):
FURABID CR CAPSULE, 100 MG. The pharmacist carries out medication monitoring using the information system, no (warning) signals are given.
The pharmacist carries out pharmaceutical care (compliance, education, et cetera) and agrees with the patient on how she will use the medication. This TA on 27 January yyyy reads:
FURABID CR CAPSULE, 100MG; 1 capsule 2x daily; from now on for 5 days.
The pharmacist supplies the medication to the patient:
on 27 January yyyy, FURABID CR CAPSULE, 100 MG; 10 capsules.
The TA and MVE are sent to the prescriber and made available to fellow health professionals and the patient.
4.2.2 New medication agreement, more precise product specification
Here, the difference when specifying the product is that the TA and also the MVE deviate from the MA the patient has made with the prescribing physician. The process is otherwise the same as described in the previous paragraph.
Example
MA: Nitrofurantoin capsule mga 100 mg, 1 capsule 2 times a day from today for 5 days.
The pharmacist specifies a different product:
Nitrofurantoin MC Actavis capsule 50 mg.
The pharmacist agrees with the patient on how she will use the medication. This TA on 27 January yyyy reads:
Nitrofurantoin MC CR capsule 50 mg, 1 capsule 4x daily, from now on for 5 days.
The pharmacist supplies the medication to the patient:
on 27 January yyyy, Nitrofurantoin MC Actavis 50 mg; 20 capsules.

4.2.3 Existing administration agreement is adequate
The process of repeat MVEs on the basis of an existing MA and possibly existing VV and TA occurs in case of repeat prescriptions and is described in paragraph 4.2.10, situation 2.
4.2.4 Proposal to prescriber for medication agreement
Example 1 - adjusting dosage:
For a 70-year-old patient with a creatinine clearance of 45 mL/min, the pharmacist enters a TA for gabapentin tablets of 600 mg, 1 tablet 3x daily. Renal function has been recorded in the patient’s file at the pharmacy, and based on this a signal appears that the maximum dosage is 900 mg daily. The pharmacist sends the prescriber a VMA with an adjusted dosage of 300 mg, 3x daily, and the reason for this proposal (maximum dosage exceeded). The prescriber receives the VMA in his EVS. The prescriber sends an adjusted MA and possibly an AVMA back to the pharmacist.
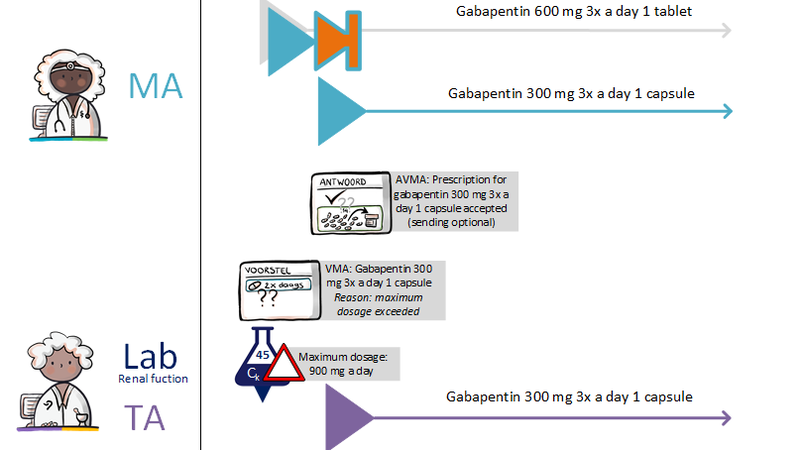
Example 2 – temporarily halting a medicinal product:
For a 50-year-old patient with on oral fluconazole as part of his current medication, the pharmacist enters a TA for simvastatin. A message appears with the advice to consider temporarily halting simvastatin. The pharmacist can make a VMA for halting the simvastatin (stop type ‘suspended’). See example 1 for further steps.
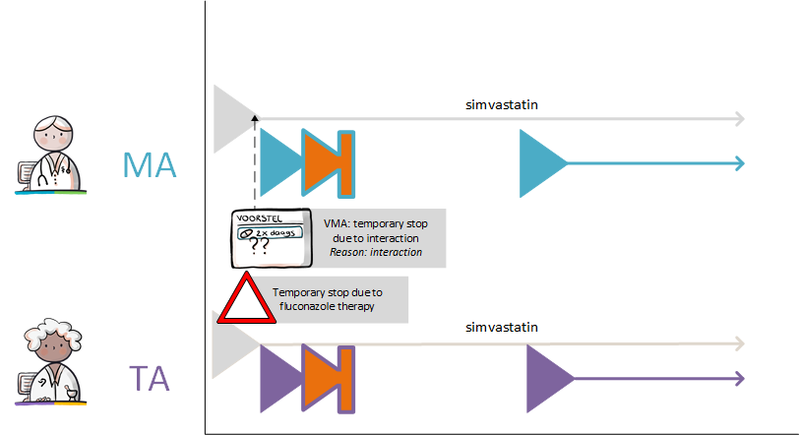
Example 3 – adding a medicinal product:
For a 55-year-old woman, the pharmacist enters a TA for prednisone, 10 mg daily for thirteen weeks. The patient is not using osteoporosis prophylaxis. A message appears with the advice to add a bisphosphonate drug and to check whether the patient is taking calcium and vitamin D. If the patient is not, the pharmacist can make a VMA for these products. See example 1 for further steps.
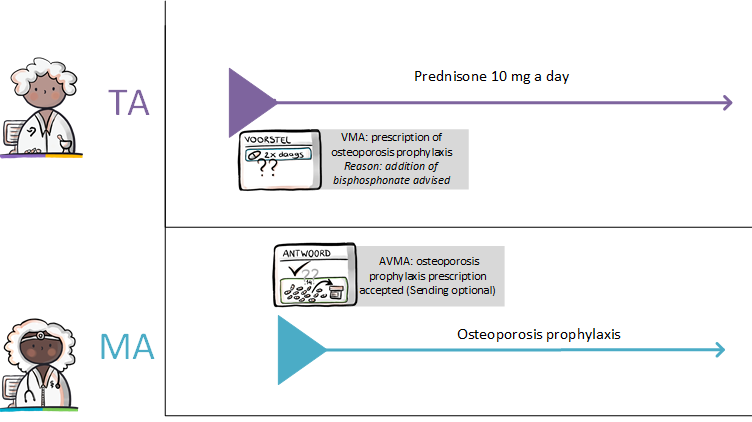
PLEASE NOTE: it will depend on the situation whether it is useful to create an automated proposal for adjustment/addition of an MA. If the advice is complex, it may still be useful to phone and to consult instead of sending a digital proposal.
The VMA may help the prescriber to enter this change in the system. The latter is important when MAs are consulted, for example, by service observation general practitioners.
4.2.5 Request and dispense
It is possible to create multiple MVEs under the same TA:
- A VV comes in with an MA with a one-year use period
- There will be a first MVE with a new TA and a medication supply that is sufficient for, for example, a period of 4 months.
- The second MVE is performed based on the existing MA, VV and TA (no new prescription or new TA is needed). The new second MVE (with new RequestDate and the pre-existing associated TA) is sent to the prescriber when the MedicationDispenseDateTime is registered. See paragraph 8.3.
4.2.6 Patient requests repeat prescription via physician (reactive repeat)
A physician has previously diagnosed hypertension in a patient (57 years old) and has prescribed the patient medication for this. The patient uses this medication and the medication is running out. The patient makes a request to repeat the medication, for example through the repeat line, by phone, by email to the practice, counter/boxes, website, app or portal of the physician. The physician approves the repeat. This leads to a new VV.
For example:
The general practitioner makes, in the context of the MA of 30 March yyyy:
- Nifedipine CR 30 mg; 1 tablet 1x daily, from 30 March yyyy for an indefinite period
on 13 April yyyy a VV to a pharmacist chosen by the patient:
- Nifedipine, CR tablet, 30 mg; ‘for three months’.
The prescriber sends the VV with accompanying MA to the patient's pharmacy. The prescriber also makes the new VV available for the patient. The existing MAs had already been made available.

The pharmacist receives the VV together with the MA and processes it in accordance with the dispense process and starts with the pharmaceutical care process step.
When the patient, on the basis of a TA or on the basis of his MGB asks for a repeat prescription, and the MA is not digitally available, the physician creates a new MA based on this TA or the patient’s MGB of the medication. In that case, no RelationMedicationAgreement will be registered.
4.2.7 Patient requests repeat prescription via pharmacist
The patient requests a repeat medication from the pharmacist, for example through repeat line, by phone, email, counter/boxes, website, app or portal of the pharmacist.
The pharmacist sends a VVV for authorisation to the prescriber. The physician evaluates the VVV and approves it. He sends a new VV with accompanying MA, and possibly an AVVV to the pharmacist. The pharmacist continues the dispense process and starts with the pharmaceutical care process step.

If the prescriber does not issue a VV, he lets the pharmacist know by sending an AVVV.
4.2.8 Proactive repeat prescription by pharmacist
The patient has signed up in the past for proactive repeats and the pharmacist has registered this in his own pharmacist information system. The repeat module of this information system will generate a signal when the patient needs new medication. When a new VV is needed, this is similar to the process described in the previous paragraph, only the trigger is not the patient but the pharmacist (or his information system). Proactive repeats are also used for GDS, see paragraph 4.2.11.
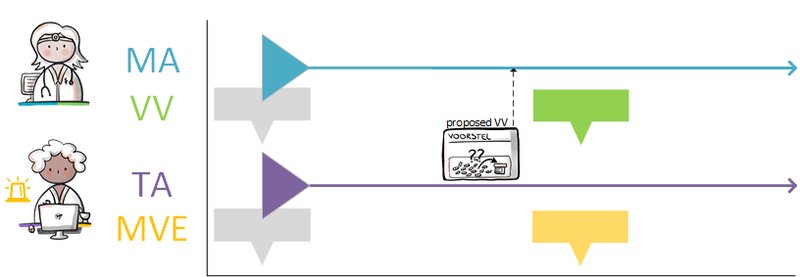
4.2.9 Dispense based on an existing dispense request
The patient submits a repeat prescription to the pharmacist. In this case, it is a request for dispense of mediation on the basis of an existing, still valid, VV. See paragraph 4.2.10 situation (3).
4.2.10 Splitting a prescription
The patient has been using the same medication for a number of years, which means that the MA can be created for an indefinite period. The TA is also valid for an indefinite period. In such a case, the patient can be given a prescription for a year. This single year prescription will be split into several prescriptions by the pharmacist. These split prescriptions are commonly called ‘repeat prescriptions’, even though strictly speaking this is not true.
There are three possible situations:
1) The patient returns to his attending physician still suffering from the same symptoms. The prescriber prescribes the same medication (in the case of an expired MA, a new MA is created with a new VV and in the case of an MA that is still valid, only a new VV is created).
2) Prescriber and patient initially agree on 90 tablets with 3 repeat prescriptions. The patient receives the first 90 tablets. The next time the patient visits the pharmacist, an MVE is made under the existing agreements.
3) Prescriber and patient initially agree on 360 tablets for one year. The pharmacist splits this into 4 separate occasions of MVE. The patient is initially supplied with 90 tablets and without a new VV, can go to the pharmacist 3 more times to pick up another 90 tablets each time.
Situation 3 best describes the way in which a pharmacist splits a year prescription.

4.2.11 Starting and continuing a GDS
The following example shows the process for one medicinal product used by the patient for an indefinite period of time. The information system of the prescriber and the pharmacist contains (among other things) the following data on a patient:
- The MA of 2 January yyyy reads: Metoprolol, CR tablet, 100 mg (succinate); 1x tablet daily; from 2 January yyyy for an indefinite period.
- The TA of 2 January yyyy reads: Metoprolol PCH retard, 100 CR tablets of 95 mg; 1x tablet 1x daily; from 2 January yyyy for an indefinite period.
This elderly patient is taking so much medication that he has problems overseeing it all: a potentially dangerous situation. On 6 February, the pharmacist and the prescriber agree that the supply of medicinal products to this patient will take place via GDS.
The GDS is started: the pharmacist sends a VVV (a similar event to the current combined prescription or authorisation form).
The VVV of 6 February reads:
- VV for the MA of 2 January yyyy: Metoprolol, CR tablet, 100 mg (succinate), and a PeriodOfUse to ensure enough stock up to and including 1 May yyyy.
The pharmacist sends the proposal to the prescriber. The prescriber approves the proposal on February 6 and sends it unchanged to the pharmacist as a VV. The GDS indicator is switched on in the information system of the prescriber as well as in the pharmacist’s information system.
The pharmacist and the patient select a partial duration for the product. This TA reads as follows:
- On 7 February it was agreed: Metoprolol PCH retard, 100 CR tablets of 95 mg, 1 tablet in the morning, from 11 February (week 6), for an indefinite period.
Every second week, the pharmacist supplies the patient with a Baxter medication roll containing (among other things) the metoprolol:
- MVE 1: The pharmacist dispenses, on Friday, 8 February yyyyy: 14 tablets, scheduled for weeks 6 and 7 (a PeriodOfUse of 2 weeks).
- MVE 2: The pharmacist dispenses, on Friday, 22 February: 14 tablets, scheduled for weeks 8 and 9.
- MVE 3, 4, 5, etc.
With each MVE, it is indicated that dispense of medication was carried out via GDS, in data element DistributionForm.
After three months, there is a new VVV from the pharmacy to the prescriber, etc.
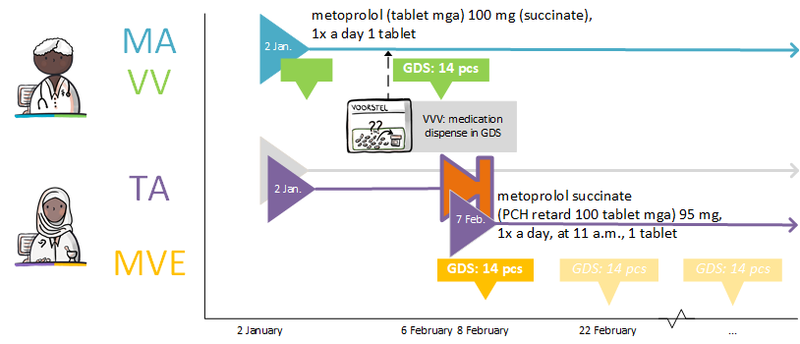
Consideration
- The application of a VVV instead of an authorisation form makes its meaning unambiguous.
- In the current situation, TAs and MVEs are registered as a single dispense. This makes it difficult to find useful information in the large number of messages. By dividing the dispense message into two building blocks, the overview is easier to understand: there are no new MAs and only one new TA is created. There are no hidden surprises in the remaining logistical data and the prescriber does not have to monitor the multiple MVEs.
- This way of registering and exchanging prevents double registration, and errors caused by this.
- The TAs are the basis for the administration list and checklists of nursing homes and care homes. Signing them corresponds to the administration(s).
4.2.12 Pharmacist changes commercial product
This scenario builds on the previous scenario: after half a year a different commercial product is agreed with the patient (TA), because the original product is not available. No new MA is made, as there is no change needed on that level. The TA is changed:
- On 1 July it was agreed: Metoprolol Sandoz ret, 100 CR tablets, 95 mg, 1 tablet in the morning; from 3 July, for an indefinite period. Reason: Drug not available - out of stock (in data element AdministrationAgreementReasonModificationOrDiscontinuation).
The TA is a modification of the TA of 7 February, which stops on 2 July (11:59 p.m.) and is an actual handling of the MA of 2 January. The pharmacist sends the new TA and any MVE to the general practitioner.
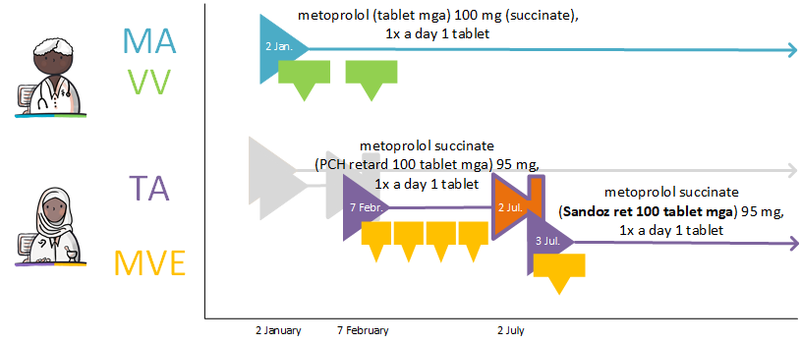
Consideration
This change in the actual handling of the MA using a TA, changes nothing in the validity of the MA itself, improving the overall view for all parties. In addition, since the pharmacist records and communicates the reason for the adjustment, the prescriber is better able to assess whether the information is relevant.
4.2.13 Adding medication to a GDS
When new medication is added to an existing GDS, the prescriber may opt for immediate addition to the current roll (Change in GDS immediately) or for addition starting from the next roll (Change in GDS per change of roll). The prescriber indicates this in the data element AdditionalInformation in the MA.
If ‘Change in GDS immediately’ is chosen, the pharmacist will first supply the medication separately, in addition to the existing GDS. This means that a TA is created by the pharmacist for the bridging period. Starting with the new roll, the new medication is added to the roll. Starting with the startDateTime of the new roll, a TA can be made by the pharmacist with specific administration times. If the ‘bridging-TA’ already includes the correct administration times, no new TA is created at the date of inclusion in the roll.
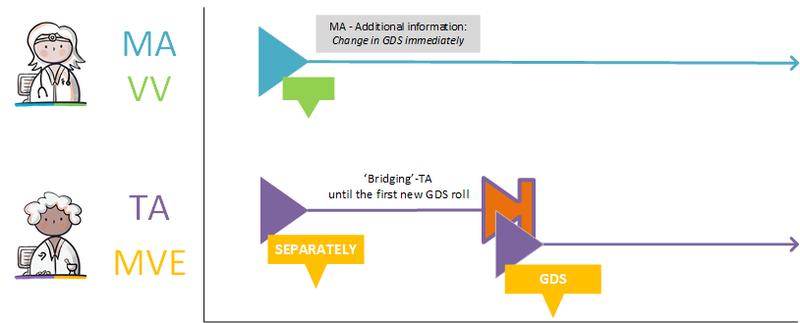
If 'Change in GDS per change of roll' is chosen, the pharmacist will start to include the medication in the roll on the startDateTime of the first new roll. In this case, a TA for the bridging period is not required.
4.2.14 Discontinuing medication in a GDS
When medication is discontinued, the prescriber creates a stop-MA. The stop-MA is sent to the pharmacist who can adjust the supply accordingly. The pharmacist will create a stop-TA. See paragraphs 2.2.5.3 and 2.3.6.3.
4.2.15 GDS supplier supplies other commercial product
It is possible that a GDS supplier delivers a different commercial product than that which is included in the TA by the pharmacy. This is therefore a change in the HPK which results in a changed TA. This may be done only afterwards, after feedback from the GDS supplier of the filling details.
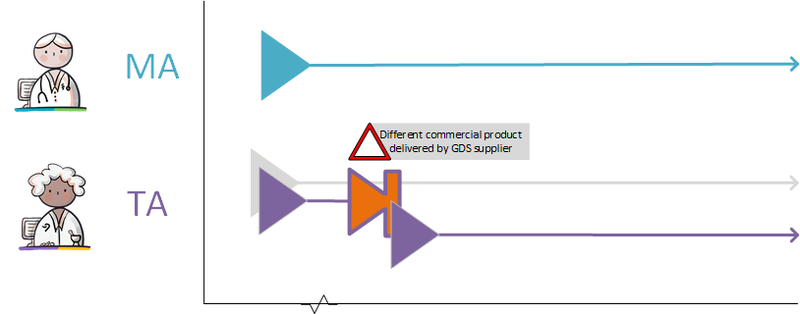
4.2.16 Parallel administration agreements with GDS and non-GDS dispense
A patient takes two to three times daily 20 mg pantoprazole because of gastric complaints. The first two tablets are fixed dosages, which are supplied in GDS. The third tablet, which is prescribed as needed, is supplied separately. At a certain moment, the pantoprazole 20 mg from Sandoz is no longer available for separate supplies. Therefore, the pharmacist decides to supply pantoprazole from Apotex. He stops the current TA and splits the TA in two TAs, both with the same startDateTime. The first TA concerns the fixed dosage in GDS, the second TA involves the as needed tablet.
NB. Currently, there is no relationship between medication dispense and TA. Therefore, it is only possible to retrieve which TA is associated with the medication dispense by assessment of the data content. Whether the functional design for this situation should be adjusted in the future is being evaluated.
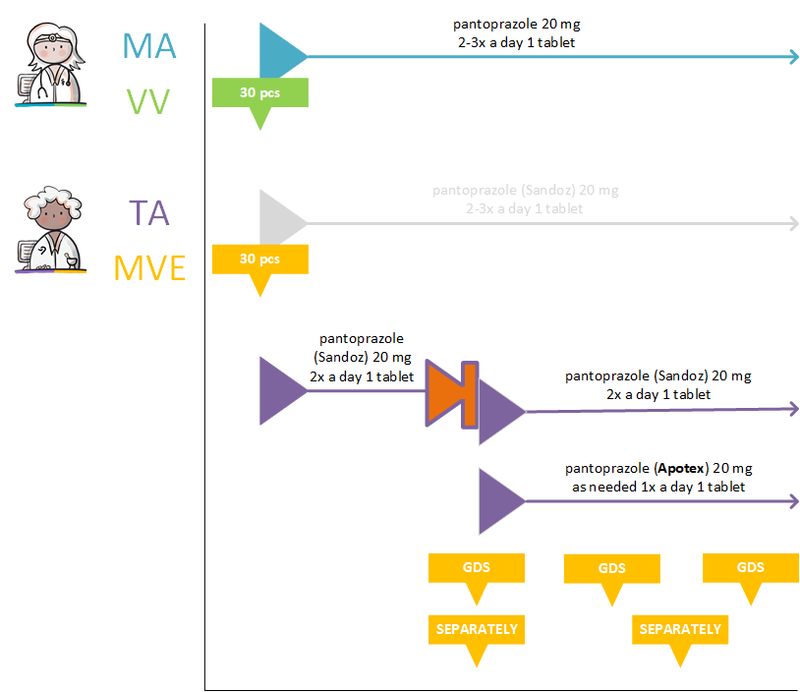
4.2.17 Parrallel administration agreements of which one is changed
A patient visits the general practitioner with stomach complaints, upon which the general practitioner prescribes pantoprazole.
Pantoprazole tablet 20 mg; 2-3x daily.
The fixed dosage of two tablets goes into GDS are supplied by the GDS-provider of the Sandoz brand. The third tablet is dosed ‘as needed’ and is provided separately by the pharmacist from the Apotex brand by the patients regular farmacy. This results in two parallel TA’s. At a certain point, the GDS supplier can no longer supply pantoprazole. The pharmacy does have stock of Apotex and adjusts the GDS TA for the fixed dose to a separate dispensing of 1 tablet of 20 mg Pantoprazole Apotex twice a day. Stopping the GDS TA does not effect the ‘as needed’ TA. This creates two parallel TA’s that are both dispensed separately by the pharmacist. The one containing the fixed dose and the other the ‘as needed’ dose. In this way, they remain visible separately on the administration list as fixed dose and ‘as needed’ dose.
4.2.18 Handling a stop-medication agreement
When the prescriber agrees to discontinue medication, a stop-MA is created. This stop-MA is processed by the pharmacist by discontinuing the corresponding TA.
4.2.19 Dispense with someone else’s administration agreement
A patient takes two times daily 20 mg pantoprazole because of gastric complaints. During the holidays the patient notices that he has forgotten to bring his medication. He visits the after-hours general practice clinic and asks for extra tablets for during his holidays. The after-hours physician sends a VV to the after-hours pharmacy, using an existing MA.
The after-hours pharmacist reads the medication prescription and creates an MVE, using the existing TA, for the ten tablets that the patient needs. The pharmacist notifies the after-hours physician about the medication dispense, and sends a copy of the original TA.
The VV and MVE are made available to fellow health professionals (including the original prescriber and dispenser) and the patient.
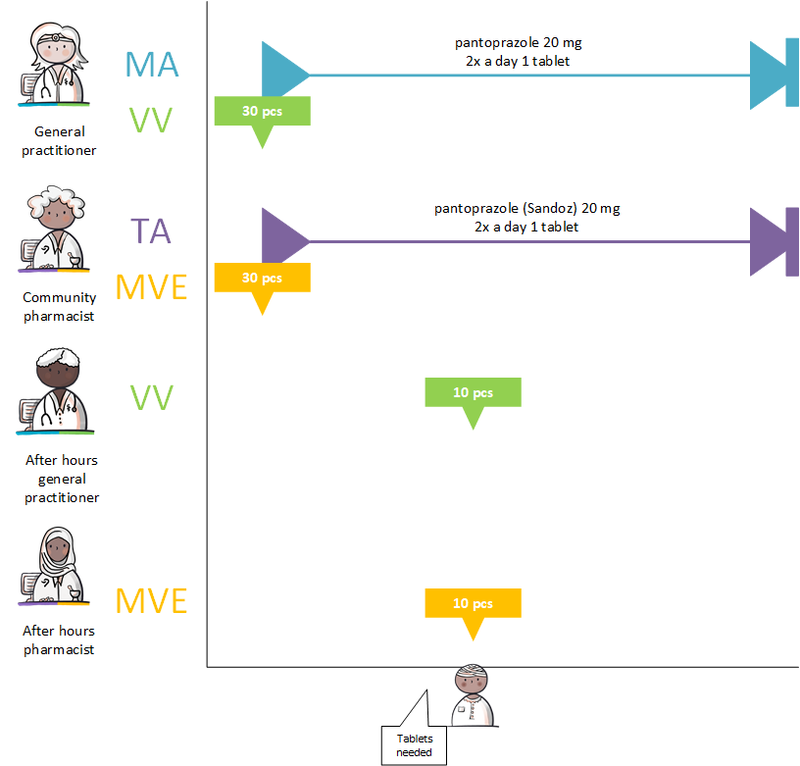
4.2.20 Modification of someone else’s administration agreement
A patient takes two times daily 20 mg pantoprazole because of gastric complaints. During the holidays the patient notices that he has forgotten to bring his medication. He visits the after-hours general practice clinic and asks for extra tablets for during his holidays. The after-hours physician sends a VV to the after-hours pharmacy, using an existing MA.
The after-hours pharmacist notices that they have no pantoprazole from Sandoz in stock, but they do have pantoprazole from Apotex. Therefore, she stops the current TA, starts a new TA for the Apotex, and creates an MVE for the ten tablets from Apotex. The after-hours pharmacist sends the stop-TA, the new TA and the MVE to the initial pharmacist. The latter processes the stop-TA in his own information system.
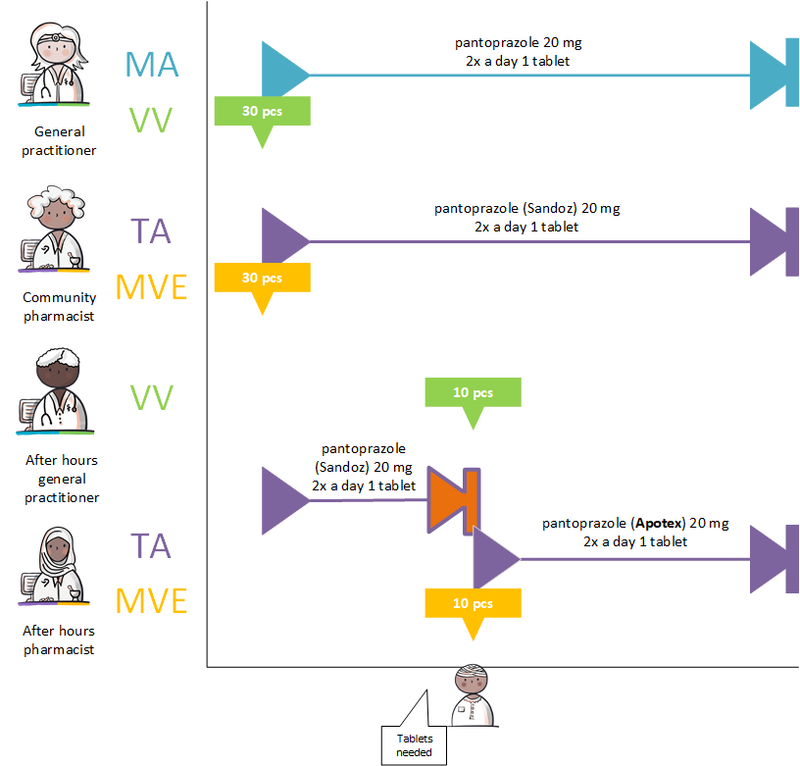
4.2.21 Deviation from prescribed quantity in DispenseRequest
For a patient with stomach complaints, the general practitioner prescribes pantoprazole 1x per day 1 capsule for 28 days and sends a DispenseRequest to the pharmacist for 28 pieces. The pharmacist only has packaging of 30 pieces. There are two capsules left at the end of the course, but the MA can still be met. A TA for 28 pieces follows. The MVE states that 30 units have been delivered, optionally with an explanation as to why this deviates from the TA, but this is not mandatory.
For a patient with stomach complaints, the general practitioner prescribes pantoprazole 1x per day 1 capsule for 30 days and sends a request for dispensing to the pharmacist for 30 pieces. The pharmacist only has packaging of 28 pieces. The pharmacist contacts the prescriber based on professional judgment. There will be a provision of 28 pieces with a TA for 28 days. The TA and MVE deviates from the MA the patient has made with the general practitioner. The pharmacist's information system makes the TA and MVE available to the patient's co-practitioners.
4.3 Practical examples, Administer
4.3.1 Creating an administration list
When an administration list has to be created, for example when a patient starts to receive medication administered by a (professional) administrator, or when GDS medication or non-GDS medication is started, an intake is planned with the patient and the prescriber, pharmacist or administrator (the exact process may differ across institutions). Then, the prescriber, pharmacist, administrator and/or the patient consult on the administration list (which medication is recorded on the administration list, which (guide) administration times are suitable). The pharmacist records the (guide) administration times in the TA and sends the required medication data for the administration list to the administrator and/or patient and/or makes these data available to them.
4.3.2 Exact administration times required
When exact administration times are required, for example because of medication interactions, the prescriber or the pharmacist can indicate that an exact administration time is required (the administration time is not a guideline but denotes an exact time). The prescriber or pharmacist can register an exact administration time by indicating that the administration time is not flexible in the data element IsFlexible under AdministeringSchedule in the MA or TA. By default, administration times are flexible. Exact administration times should be presented on the administration list, to ensure that the administrator is informed that it is not allowed to deviate from the entered administration time.
4.3.3 Missing (guide) administration times
When (guide) administration times are missing in the MA and TA, an administrator will contact the prescriber or pharmacist to retrieve the (guide) administration time. This is not supported digitally in this information standard.
4.3.4 Non-GDS medication as needed
Non-GDS medication as needed is included in the administration list, but the administration moment and/or time is not available beforehand. If relevant (for example because of drug interactions), (guide) administration times can be entered.
See the following practical examples: 4.1.4 Medication as needed, 4.1.5 Course of treatment as needed starting in future, 4.1.6 Two dosages of the same medication at the same time, 4.1.7 The same medicinal product with different strengths at the same time.
4.3.5 Medication supply by multiple pharmacies
Multiple pharmacies may be involved in the medication supply for one patient, for example in the following situations: medication supply by an outpatient pharmacy, in addition to supplies by a community pharmacy or supplies of add-on medication by a hospital pharmacy. In the case of medication supply by multiple pharmacies, each pharmacist is responsible for the medication data that is required for the administration list, including the (guide) administration times in the TA. It is the responsibility of the prescriber or pharmacist who has made the most recent medication adjustments to determine whether an addition/modification is appropriate with regard to the total of (known) medication data; this concerns both medication monitoring and (guide) administration times. Because the medication details from all pharmacists involved have been made available, they can all be shown in one administration list.
4.3.6 Change in GDS from the next supply or immediately
If GDS medication is altered, it depends on the situation whether this change should occur immediately or from the next supply. The prescriber can indicate in the MA when the change in GDS medication should be implemented. An immediate modification should be immediately shown on the administration list, to ensure that the (professional) administrator administers the correct (amount of) medication. In contrast, a modification that should be implemented from the next supply, should not be processed immediately in the administration list, since previous agreements remain valid until the next supply. Thus, the administration list should not be modified until the change in GDS modification has become effective.
See also practical example 4.2.13 Adding medication to a GDS.
4.3.7 Increasing dosage of GDS in new MBH
A patient is administered 1 tablet propranolol 40 mg 1 time a day. Due to insufficient effects, the prescriber decides to increase the dosage to 80 mg 1 time a day. A new MBH is created for this MA, as there is a change on prescription level. However, the patient still has the 40 mg tablets in the GDS medication roll for the coming days. Therefore, the pharmacist decides to supplement the GDS medication with the administration of 1 tablet propranolol 40 mg, separately from the GDS package, in addition to the tablet in the GDS package, until the next GDS medication roll change in 5 days. The pharmacist creates two TAs: one for the previous GDS medication and one for tiding over the period with a medication dispense separate from GDS. In the new GDS supply (medication roll change), the 80 mg tablets are incorporated in this new GDS package.
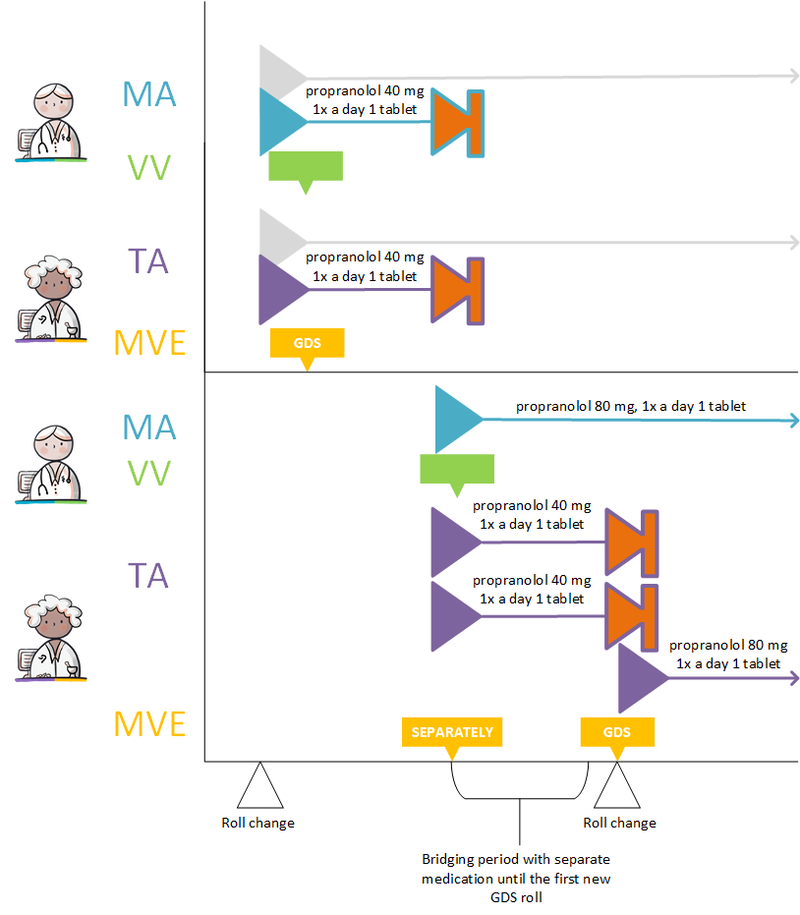
4.3.8 Decreasing dosage of GDS in new MBH
A patient is administered 1 tablet propranolol 80 mg 1 time a day. Due to a too strong effect, the prescriber decides to reduce the dosage to 40 mg 1 time a day. A new MBH is created for this MA. The patient still has 80 mg tablets in the GDS medication roll for several days. Because the administrator is not allowed to divide the tablets into halves, the supplier decides that the remaining GDS packages should be collected the same day and be replaced by a new GDS medication roll with 40 mg tablets.
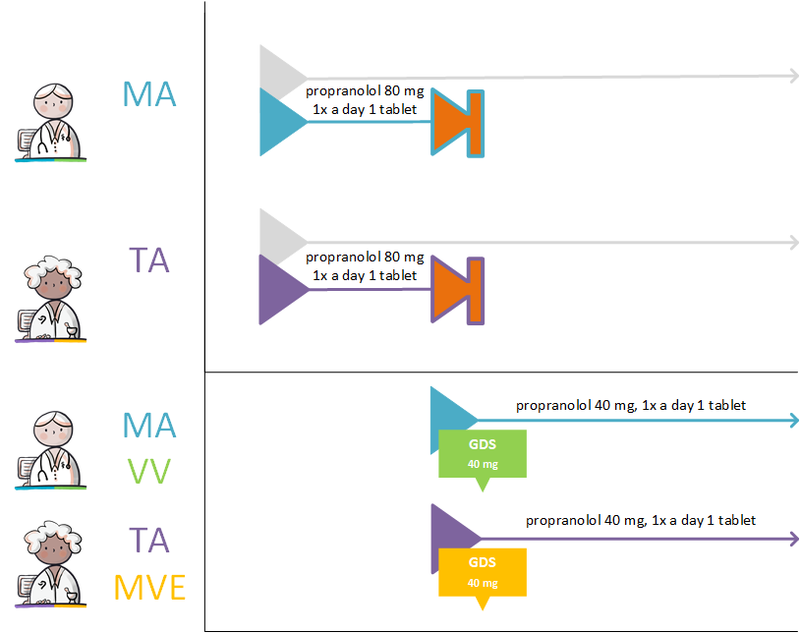
4.3.9 Change processed by the pharmacist
A change by the prescriber, such as starting new medication, adjusting the dosage, (temporarily) halting medication, is recorded by the prescriber in the MA and/or the VV. Then, the pharmacist processes this change in the TA and/or the MVE. This may be done during the opening hours of the community pharmacist, or after hours when a medication supply is needed. The after-hours pharmacist supplies the medication; in this case, multiple pharmacists are supplying medication (see also paragraph 4.3.5). To prepare the administration list, the medication data are sent and/or made available to the administrator by the prescriber(s) and pharmacist(s). Requesting medication data for the purpose of drawing up a administration list is preferably done automatically. Recently stopped medication administrations are also shown in the administration list.
4.3.10 Change not processed by the pharmacist
When medication is changed by a prescriber after the opening hours of the community pharmacist and no medication supply is needed, no pharmacist is involved. In this case, the MA sent and/or made available by the prescriber is leading. The administrator will be informed of the changed or stopped MA; this MA overrules all medication building blocks within the same MBH. Recently stopped medication administrations are also shown in the administration list. Requesting medication data for the purpose of drawing up a administration list is preferably done automatically. This situation also applies if the pharmacist has not processed the modification by the prescriber yet. The pharmacist is responsible for adjusting the TA as soon as possible.
4.3.11 Extra instructions for administering, explanation in MA, TA and/or WDS
Extra instructions regarding the administration of medication can be provided to the (professional) administratior and/or to the patient. An example is the situation when fentanyl patch therapy is stopped; the patch has to be removed. Another example is the dosing of half a tablet; there has to be an instruction on what should be done with the other half. These extra instructions are registered as free text in the data element Comment in the MA, TA and/or WDS.
4.3.12 Medication administration deviates from administration list
It may happen that the administration deviates from the instructions as entered by the prescriber and pharmacist in the MA or TA. This may be a deviation in AdministrationDateTime, AdministeredAmount, RouteOfAdministration, AdministeringSpeed, AdministrationProduct or no administration at all. A (professional) administrator can deviate from the rules, in consultation with the prescriber, for valid reasons, and under the condition of safe medical practice and safe working practice. The reason for deviation can be entered in MedicationAdministrationReasonForDeviation in the MTD.
4.3.13 Medication administration without medication agreement and administration agreement
In the case of an emergency, the MA and TA may be missing and, therefore, the (professional) administrator has to administer medication on the basis of an MA which is communicated verbally/by telephone. This medication is not incorporated in the administration list. In this situation, the (professional) administrator records the MTD in the information system of the administrator (including ECD, TDR). This is the first building block in a new pharmaceutical treatment; therefore, the administrator creates an MBH.
4.3.14 Medication administration of self-medication
According to the safety principles in the medication chain, the administrator is not concerned with the administration of self-medication, except for prescribed self-medication (a prescriber has created an MA). In that case, the self-medication is listed on the administration list as GDS medication or non-GDS medication (as needed).
4.3.15 Correction of an administration
This concerns correcting or cancelling an MTD, because an administrator has made a mistake in the registration and the administration is different from what has been registered.
If the MTD has not yet been sent and/or made available to other healthcare providers, the administrator can adjust the MTD by himself, or remove (cancel) the registration from his own information system.
If the MTD has already been sent and/or made available to other healthcare providers, a correction can be made by sending and/or making additional medication administrations. Below two examples of corrections are described.
Correction with negative AdministeredAmount
A patiënt is prescribed pantoprazole 40mg by their GP. Due to issues with logistics, the pharmacy hands out pantoprazole 20mg, and instructs to take 2 tablets per day. The patiënt receives support for taking her medication by a home case organisation. The home care worker gives 1 tablet to the patiënt. After registration of the administration, she notices two tablets should have been given. She administers the second tablet and corrects the medication administration. This can be exchanged as an MTD with -1 tablet to nullify the first MTD, and consecutively an MTD with 2 tablets given.
Correction with additional MTD
A client has to take 1000mg calcium due to osteoporosis. They receive help from a home care worker once per day. The pharmacy delivers calcium tablets of 500mg with the instruction to take two tablets per day. The tablets are quite large so the client can only take them separately. The home care worker administers 1 tablet calcium 500mg and registers this in an MTD. after a few minutes the second tablet is administered. The home care worker corrects the MTD by registering another MTD of 1 tablet calcium 500mg.
4.3.16 Medication not administered
A patient is given 1 tablet of propranolol 80mg every day. The patient has lower blood pressure than normal due to fever. The (professional) administrator contacts the prescriber, and in consultation it is decided to not administer the medication that day. The reason is recorded in MedicationAdministrationReasonForDeviation in the MTD.
4.3.17 Medication administration on hold
It may happen that the medication administration was unsuccessful, but that is still possible to administer the medication at a later moment, in consultation with a prescriber or pharmacist. If the medication administration is in keeping with the agreements on flexible administration times, the administrator is allowed to deviate from the (guide) administration time.
An example of suspending the medication administration:
- Medication administration is unsuccessful, but the medication can be administered at a later moment. First, an MTD can be registered with an AdministeredAmount of 0, and possibly a MedicationAdministrationReasonForDeviation. Then, if the medication is administered at a later moment, this is registered in a new MTD, possibly with a MedicationAdministrationReasonForDeviation.
4.3.18 Medication administration by a prescriber
A prescriber, like a (professional) administrator, can administer medication to a patient, and record this procedure in an MTD.
4.3.19 Multiple administration organisations
Multiple administration organisations may be involved in a patient’s medication administration. For example, medication may be administered by another organisation during the evening/night than during the day. If multiple administration organisations are involved in providing patient care, these health professionals can consult each other’s MTDs to track the process of medication administration. The acts of one organisation can affect the process of another organisation (for example, has medication been administered, have there been deviations from the administration list, etc.). Below an example of such a situation is described.
A patient with renal failure must visit the hospital three times a week for renal dialysis. In addition, the patient receives home care in the morning and evening for assistance with medicine intake. Both at home and in the hospital, the patient is administered medication. Due to a bacterial infection, the patient is prescribed an antibiotic treatment by the general practitioner. Ciprofloxacin tablet 500mg; 2x a day; for 10 days
To prevent blood clotting during dialysis, the patient is given nadroparin via the dialysis line in advance in the hospital. Nadroparin injection into the arterial line 5700 IE / 0,6 ml; 1x prior to dialysis
The administer consults the current overview with the patient's medication administration and the patient's other MTDs to track the process of medication administration of the last 24 hours. The administration organisation itself is responsible for what is or is not administered at that time within its own organization.
4.3.20 Feedback to patient through a medication adherence app
In this example the patient uses an app on his mobile phone to help manage his medication. The patient or the pharmacist has created an administration schedule with reminder times (where possible using app functionality to suggest the times of administration based on the MVEs or TAs). When it is time to take his medication, the patient receives a reminder signal from the app. The patient registers the MTD and thus indicates whether he has taken the medicine or not (for instance because he forgot to bring it along with him). If the app is linked, for example, to the patient’s PGO (personal health record), medication adherence is tracked automatically.
4.3.21 Registration (stop-)MA retroactively with interim MTD
The patients is taking propranolol and suffers more and more from the side effects during the weekend. In consultation with the prescriber, a decision is made on Sunday morning to stop the medication. However, the registration of stopping the Medication Agreement, the stop MA, is only done on Monday. As a result, during the administration round on Sunday evening, the administrator is not yet aware that the MA was stopped and another medication administration takes place. Since the registration date is known, it is still possible to find out how this administration could have taken place. The MTD is a registration of an action that has taken place. A stop MA or TA therefore has no influence on the validity of an MTD.
4.3.22 Registration of separate MTDs per InjectionPatchSite
A patient uses long-acting insulin Glargine 50 units a day in the evening. Every evening, an healthcare worker comes every evening to administer the insulin. Because of the high amount of insulin that has to be administered, the administration must be done in two administrations. The administration list shows 1 line for abasaglar 50 units. The healthcare worker first administers 25 units with InjectionPatchSite ‘left upper leg’ and MedicationAdministrationReasonForDeviation ‘2 administrations in relation to 50 units’. Thereafter, the healthcare worker administers another 25 units. In this MTD, the healthcare worker registers the ‘InjectionPatchSite ‘right upper leg’.
4.3.23 Patient transfer to a different department with the same GDS supply date
A patient is transferred from one department to another. At the current department the patient has medication supplied through GDS packaging. The date for the next GDS supply at the new department is the same as the supply date of the first department. Good communication between the departments is required in order to ensure correct medication administration on the day of the transfer, and correct resupply of GDS packaging at the new department.
4.3.24 Patient transfer to a different department with an earlier GDS supply date
A patient is transferred from one department to another. At the current department the patient has medication supplied through GDS packaging. The date for the next GDS supply at the new department is earlier than the supply date of the first department. Good communication between the departments is required in order to ensure correct medication administration on the day of the transfer, correct resupply of GDS packaging at the new department and the recollection of the GDS packaging from the first department.
4.3.25 Patient transfer to a different department with a later GDS supply date
A patient is transferred from one department to another. At the current department the patient has medication supplied through GDS packaging. The date for the next GDS supply at the new department is later than the supply date of the first department. Thus, the patient will be out of medication before the GDS supply at the new department. The pharmacy will have to supply this medication separately in the meantime.
4.3.26 GDS medication dosage increase, no change in administration times (option A)
Due to high blood pressure, one tablet of propranolol 80mg is administered to the patient every morning at 8:00. Due to a lack of drug action the prescriber decides to increase the dosage to two tablets 80mg propranolol every morning at 8:00. The dosage increase has to happen immediately and cannot wait for the next GDS supply. One option (A) in this situation is to supply separate medication in parallel with the GDS medication until the next resupply takes place. This depends on the degree of independence of the patient. The administration times do not change in this case. The higher dose is supplied in the next GDS packaging.
4.3.27 GDS medication dosage increase, no change in administration times (option B)
Due to high blood pressure, one tablet of propranolol 80mg is administered to the patient every morning at 8:00. Due to a lack of drug action the prescriber decides to increase the dosage to two tablets 80mg propranolol every morning at 8:00. The dosage increase has to happen immediately and cannot wait for the next GDS supply. One option (B) in this situation is to recollect the previous GDS packaging and supply the full dose separately outside of GDS packaging until the next resupply. This depends on the degree of independence of the patient. The administration times do not change in this case. The higher dose is supplied in the next GDS packaging.
4.3.28 GDS medication dosage increase, change in administration times
Due to high blood pressure, one tablet of propranolol 80mg is administered to the patient every morning at 8:00. Due to a lack of drug action the prescriber decides to increase the administration schedule to one tablet 80mg propranolol every morning at 8:00 and one tablet every afternoon at 16:00. The frequency increase has to happen immediately and cannot wait for the next GDS supply. In this situation the previous GDS packaging has to be recollected and the full dose is supplied separately outside of GDS packaging until the next GDS resupply. The administration times have changed in this case. The higher dose is supplied in the next GDS packaging.
4.3.29 GDS medication dosage decrease
Due to high blood pressure, once per day two tablets of propranolol 80mg are administered to the patient. Due to too strong drug action the prescriber decides to decrease the dosage to one tablet 80mg propranolol per day. The dosage increase has to happen immediately and cannot wait for the next GDS supply. In this situation the previous GDS packaging has to be recollected and the new dose is supplied separately outside of GDS packaging until the next GDS resupply. The dosage decrease is supplied in the next GDS packaging.
4.3.30 Changing GDS medication to ‘as needed’ medication
The prescriber makes a prescription of temazepam to help insomnia caused by stress. The symptoms are decreasing so the prescriber decides to change the prescription from once per day 10mg temazepam to once per day 10mg temazepam as needed. The change has to take effect immediately and cannot wait for the next GDS resupply. This means that from now on the medication has to be supplied outside of GDS packaging and the current GDS packaging has to be recollected by the pharmacy.
4.3.31 Splitting GDS medication into GDS medication and ‘as needed’ medication
The prescriber makes a prescription for diazepam 5mg twice per day to help with anxiety. Due to too strong drug action the prescriber changes the prescription to diazepam 5mg once per day, and diazepam 5mg once per day as needed. The change has to take effect immediately and cannot wait for the next GDS resupply. This means that for now the medication has to be supplied outside of GDS packaging and the current GDS packaging has to be recollected by the pharmacy. The dosage decrease is supplied in the next GDS packaging, and the as needed medication is supplied separately in parallel.
4.4 Practical examples, Use
4.4.1 Self-care product
A patient has acquired Ibuprofen from the drugstore and takes 1200 mg every day. The patient enters this product as MGB, with such data as startDateTime and Dosage, and possibly the ReasonForUse.
4.4.2 Medication from abroad
A patient has been prescribed medication on holiday and is taking this. The patient enters the medication as MGB, with data such as startDateTime and Dosage, and possibly the ReasonForUse. If known, the name of the prescriber is also recorded.
4.4.3 Modification on the patient’s initiative
The patient has been prescribed propranolol. The patient is having severe problems sleeping because of this and has reduced the medication herself. The patient records MGB with the modified dosage and the startDateTime of the use of this lower dose. The patient also indicates that she initiated this change herself, in ReasonModificationOrDiscontinuationOfUse.
In the case where a patient initiates a dose reduction, the patient can keep on using the medication for a longer period than initially agreed because she still has a supply. The patient can indicate this as additional use.
In the case where a patient initiates a dosage increase, the patient’s supply may run out sooner. The patient may then have to return to the physician earlier than expected.
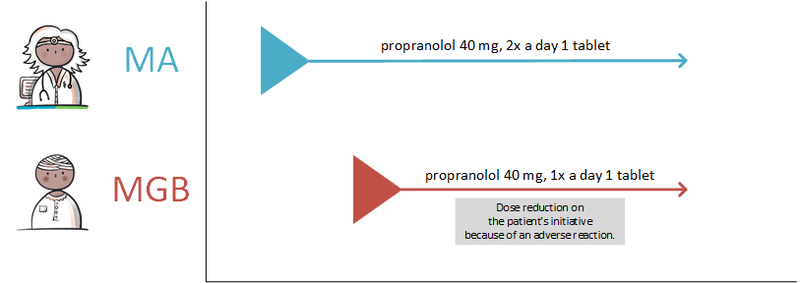
4.4.4 Discontinuation of medication on the patient’s initiative
Since he started using propranolol, the patient suffers from insomnia. He decides to stop without immediately informing the prescribing physician. The patient himself records that he has discontinued medication, indicating the date of discontinuation in endDateTime, and registering Adverse reaction to drug in ReasonModificationOrDiscontinuationOfUse. In the Comment, he adds that the adverse effect concerns insomnia.
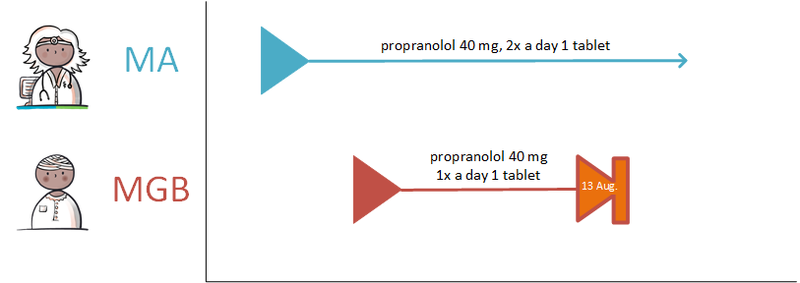
4.4.5 No more supply
A patient has received medication ‘as needed’ for his skin problems. The medication is still active in the medication profile, but is no longer available at the pharmacist. The patient indicates that the medication has been discontinued and registers Drug not available - out of stock as reason in ReasonModificationOrDiscontinuationOfUse, possibly with the addition that the symptoms have disappeared.
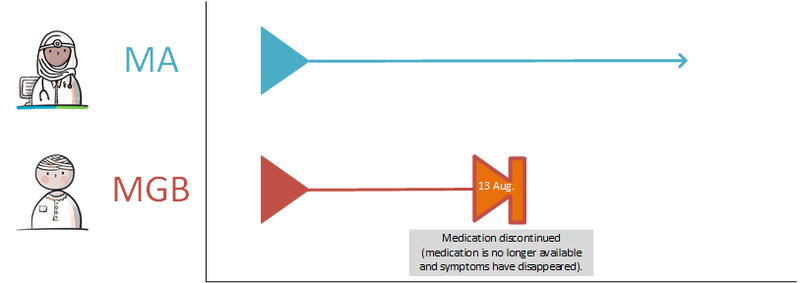
The patient may also make a VVV to replenish his supply. In this case, the AVVV comes back to the patient (and if approved, a VV is also sent from the prescriber to the pharmacist).
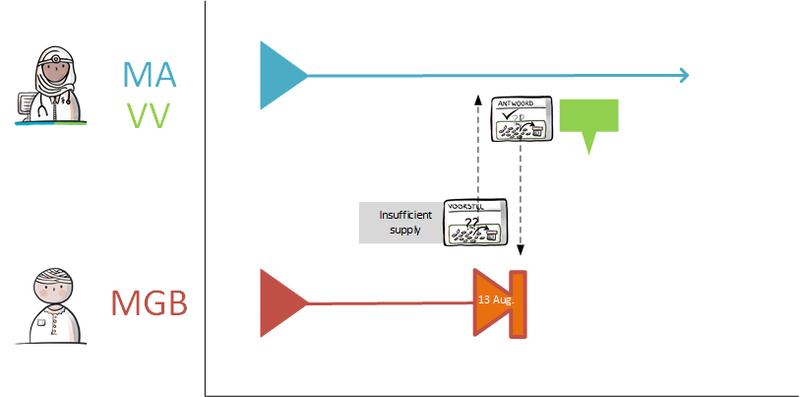
4.4.6 Registrations of abnormal medication use by patient due to adverse drug reactions
Since he started taking propranolol, a patient has been suffering from insomnia. The patient records this side effect in the explanatory notes in the MGB. When the patient changes the use of this medication from what was previously agreed, for example when he takes less tablets, he can give the side effect as a reason for this change, in ReasonModificationOrDiscontinuationOfUse. When his symptoms diminish or cease, the patient records this in the explanation in the MGB.
It is possible that the patient is suffering from side effects without exactly knowing which medicinal products cause them. There is no provision within the information standard to record this (yet).
4.4.7 Register medication use based on supply
This applies in the transition period from the old medication process standard 6.12 to this medication process information standard.
Only MVE is available digitally (according to medication process version 6.12). However, the user's information system offers the possibility to record all medication use. In that case, the MGB can be recorded with reference to the ID of the version 6.12 MVE.

5 Medication overview and inference rules
This chapter shows a sample medication overview and also specifies inference rules for 'The most current relevant building block', Verification for each MBH, etc.
5.1 Introduction
This page contains an example of a medication overview. This example was developed in the Medication Process Information Standard program and serves as an example of how data can be displayed in an application. The data numbered in the pictures below are normative, they are mandatory to show. The other (unnumbered) data in the example is optional and may be added as normative in the future. The layout of the overview in an information system can differ per target group and per device. For pharmacists, for example, only TAs are initially visible, after which it is possible to click further to get to the corresponding MA and the MGB. If only MGB or an MA is known, this will of course be shown immediately. The basic principle is that the overview shows all medication of the patient, not just the medication that is registered in the patient's own information system.
The overview is described generically and therefore the same for all suppliers. The requirements of the end users are included on this page and no statements are made about the technical realisation or implementation. The existing KNMP specification “User requirements specification Medication overview 2.0”, the “Guideline Transfer of Medication data in the chain of care, Revision 2018/2019”, and the new insights from the Medication process program have been incorporated in this chapter.
Principles for the overview are:
- Health professionals want to see a distinction between the therapeutic building blocks on a medication overview:
- MA
- TA
- MGB
- Logistical building blocks (VV, MVE) and the MTD building block are not relevant for the medication overview.
5.2 Functional specification
In the figures below the parts with normative data are included. The other parts are not yet normative. The numbered elements in the table are normative, other elements are optional for the time being.
5.2.1 Heading and General
Filter: N/A
Sort: N/A
NOTE: In the viewer, all dates are shown with the month in characters, so that there is no confusion between day-month and month-day (American format).
| No | Header | Dataset | Explanation |
|---|---|---|---|
| 1 | Name | Patient – NameInformation (Initials, LastName, LastNamePartner), DateOfBirth, Gender | |
| 2 | Telephone | Patient – ContactInformation – TelephoneNumbers | Primary telephone number of patient |
| 3 | BSN | Patient – PatientIdentificationNumber | Always BSN |
| 4 | Address | Patient – Address data | |
| 5 | Healthcare Provider Name | HealthcareProvider – OrganizationName | Healthcare Provider who has compiled the medication overview |
| 6 | Healthcare Provider Address | HealthcareProvider – AddressInformation | Healthcare Provider who has compiled the medication overview |
| 7 | Healthcare Provider Telephone | Healthcare Provider – ContactInformation – TelephoneNumbers | Healthcare Provider who has compiled the medication overview |
| 8 | Healthcare Provider Email | Healthcare Provider – ContactInformation – EmailAddresses | Healthcare Provider who has compiled the medication overview |
| 9 | Date of Medication Overview | Document data – DocumentDate | The time at which the document was created |
| Height | BodyHeight – HeightValue, HeightDateTime | ||
| Weight | BodyWeight – WeightValue, WeightDateTime | ||
| Checked by Health professional | DocumentData – VerificationHealthProfessional | ||
| Verified with Patient/Informal Caregiver | DocumentData – VerificationPatient |
Elements 5 through 8 are not shown if the patient is the creator of the medication summary.
5.2.2 Contra-indications and hypersensitivities (CiO)
Active contra-indications and hypersensitivities (intolerances, allergies/adverse reactions) (in Dutch: 'contra-indicaties en overgevoeligheden' - CiO).
Filter: endDateTime is not filled, is in the future or was less than a year ago when compiling the overview.
Sort: most recent startDateTime at the top.
This part will be elaborated at a later time and is not yet normative.
5.2.3 Current medication
Filter: all current medication, i.e. all MAs and TAs that apply at the time the overview is compiled and the associated MGB per source. Temporarily halted medication is also shown in this category. When only MGB is known, it is shown if it is not older than 13 months. When MGB is recorded by both a health professional and a patient, the last registered MGB for both is shown.
Sorting: by MBH: MA, TA, MA (recorded by patient, care provider). MBHs are sorted in descending order by startDateTime of MA, TA, MGB.
| No | Header | Dataset | ||
|---|---|---|---|---|
| 1 | Type | MA | TA | MGB |
| 2 | Medicinal product | AgreedMedicine- | MedicineForAdministrationAgreement- | ProductUsed- |
| Product – MedicationCode (if absent: ProductSpecifications, ProductName) | ||||
| 3 | Start date | startDateTime | ||
| 4 | End date/duration | endDateTime (if not available: Duration) | ||
| 5 | Dosage | InstructionsForUse – Description | ||
| 6 | Route of administration | InstructionsForUse – RouteOfAdministration | ||
| 7 | Reason | PrescriptionReason &
if applicable, ReasonModificationOrDiscontinuation |
AdministrationAgreementReasonModificationOrDiscontinuation | ReasonModificationOrDiscontinuationOfUse |
| 8 | Explanation | Comment & MedicationAgreement/AdministrationAgreementAdditionalInformation | Comment | |
| 9 | Source | Prescriber- | Provider- | Author- |
| HealthProfessional – NameInformation, Specialty | ||||
| n/a | n/a | or “Patient” | ||
5.2.4 Future medication
Filter: all medication that will become actual in the next 3 months. This includes intended use of medication. For a description of the mapping, the same applies as with the current medication, see Current medication.
5.2.5 Recently discontinued medication
Filter: any medication that has ended or stopped in the past 2 months.
For a description of the mapping, the same applies as with the current medication, see Current medication.
5.2.6 Additional lab values
Most recent lab values and abnormal renal function values.
This part will be elaborated at a later time and is not yet normative.
5.2.7 Remarks
For example, relevant (limited) health skills (competences: literacy, calculation and digital skills) that can have an impact on medication use or treatment.
This part will be elaborated at a later time and is not yet normative.
5.3 Building block instantiations
This section describes which instantiations of the building blocks belong to the medication overview. This concerns 'own' and 'someone else's' building blocks (paragraph 3.1) and then only the latest, relevant instantiations of this.
5.3.1 Own and someone else's
Both the own and a copy of (known to the sender) building blocks from other sources are included in the medication overview. This so that the medication overview is as complete as possible for the recipient. This ensures that recipients can start using it immediately and that they are not dependent on other sources. The technical representation of someone else's building block may differ from that of the real source. It is important that the recipient is aware of this. The original OID of someone else's building block should simply be given. The recipient can then choose to also collect the building block from its source in its original form.
The MA, TA and MGB building blocks have been extended with a feature that indicates whether:
- there is an 'own' building block or
- that of "someone else" (an accent building block: MA’, TA’, MGB’)
This attribute is applicable to the transactions for the medication overview. In addition, the attribute copy applies when handling a prescription, when prescribing under someone else’s MA or when changing or issuing someone else’s TA. No copies of building blocks may be delivered in other transactions.
The 'building block of someone else' attribute is on two levels:
- The template ID of an MA’, TA’, MGB’ differs from an MA, TA and MGB.
- In addition, the data element CopyIndicator is always sent with value true in MA’, TA’, MGB’.
For the medication overview, it is permitted to submit the MAs, TAs and MGB via an information system-generated MGB or MGB '(Healthcare provider is author MGB). This must have a reference to an MA, TA or MGB.
5.3.1.1 Medication overview and derivation rules
An overview of medication that the patient is using (or should be using) can be put together in an information system in two ways:
1) displaying a current medication overview (or overviews) obtained from another source (or sources)
2) showing coherent medication building blocks obtained from one's own information system and from other sources.
In both cases, this overview of medication consists of the information as included in the building blocks MA, TA and MGB.
Re 1) When this document refers to a current medication overview, this means a coherent overview in which current MAs, TAs and MGBs are included. This current medication overview is built up by the source on the basis of the data known at that time at the source. This overview can be exchanged with the transaction group Medicatieoverzicht (Medication overview) (PULL or PUSH).
The data and building blocks that are known at the source, but originating from another source, are supplied in the transaction with the 'accent' indicator so that they are recognisable as building blocks from someone else (MA', TA', MGB').
Re 2) It is also possible for an information system to compile an overview by combining own and someone else's separate individual medication building blocks. The transaction group Medicatiegegevens (medication data) (PULL) is used to request individual building blocks.
With the transaction group Medicatiegegevens (medication data) (PUSH), separate building blocks can also be sent directly to another health professional or the patient (so without requests). This happens, for example, at discharge or at the request of a co-practitioner who has the patient in front of him (e.g. patient appears to a GP or pharmacist outside the region, where this GP or pharmacist requests the data by telephone from the patient's own GP and receives it digitally).
Both Medicatiegegevens (medication data) PUSH and PULL should not provide data obtained from other sources.
5.3.2 Last relevant
Only the last relevant building blocks (per MBH) are part of the medication overview. This section describes what exactly those last relevant building blocks are for a medication overview. Firstly for prescription medication ( paragraph 5.3.2.1), then for "over the counter" medication ( paragraph 5.3.2.2). Paragraph 5.3.2.3 further discusses the special situation that, in addition to a current, a future MA within the same MBH is valid. Finally, paragraph 5.3.2.4 summarises the rules to be applied.
Note: The (technical) stop-MAs are not shown in the examples, but are implicitly assumed.
5.3.2.1 Prescription medication
5.3.2.1.1 Most simple "happy flow"
In an MBH, the simplest "happy flow" results in a sequence of:
- MA – TA – MGB
Usually, an MBH starts with an MA. A TA concretely completes the MA. MGB says something about the actual medication use of the patient in this MBH. All three building blocks are relevant in such a case and are part of the delivery of a medication overview, as indicated in green below.
- MA – TA – MGB
A TA refers to the MA it fulfills. If no referral is available in the TA, the RegistrationDateTime of the TA must be later than that of the MA to be relevant to delivery.
MGB can refer to an MA or TA. If no referral is available in the MGB, the MedicationUseDateTime of the MGB must be later than that of the MA to be relevant for delivery.
5.3.2.1.2 New medication agreement in MBH
If one creates a new MA in an existing MBH, the currently existing MAs, TAs and records of MGB are no longer relevant for the medication overview. The idea is that all older building blocks than the current MA are by definition "overruled" by this new MA and consequently no longer relevant for the medication overview.
- MA – TA – MGB - stop-MA – MA – stop-TA
If a TA and possibly also MGB are created and available following such an MA, these will be included in the medication overview.
- MA – TA – MGB – stop-MA – MA – stop-TA - TA
- MA – TA – MGB – stop-MA - MA - stop-TA – TA – MGB
The new MA can also be a stop-MA, if the patient has to stop medication permanently. This stop-MA remains relevant for the medication overview for 2 months.
- MA – TA – MGB – stop-MA - stop-TA
5.3.2.1.3 Medication use earlier than administration agreement
It is possible that you have registered MGB before the TA has been made. Here too, the idea is that the newer TA "overrules" the older registration of TA and MGB. The older MGB is therefore no longer relevant for the medication overview.
- MA – MGB
- MA – MGB – TA
Of course, a new record of MGB can then be made, which is also relevant for the medication overview.
- MA – MGB – TA - MGB
5.3.2.1.4 New administration agreement
Sometimes a change needs to be made to a TA under an existing MA. For example, because a different product has to be supplied (as a result of preference policy or for stock reasons). Again, you must create a stop-TA and then a new TA.
- MA – TA – MGB- stop-TA - TA
Of course, you can then record MGB, which in turn is relevant for the medication overview.
- MA – TA – MGB - stop-TA – TA - MGB
5.3.2.2 Over the counter
Sometimes a patient uses medication for which there is no prescription. This applies, for example, to painkillers such as paracetamol or ibuprofen, which are available without a prescription. We call this “over the counter” medication. Such use of "over the counter" medication can be recorded with the MGB building block. This is also relevant for the medication overview.
- MGB
A new record of MGB by the same type of author (type of author is patient or health professional) "overrules" any older records of MGB. Elaborated below, in order of MedicationUseDateTime:
- MGB - MGB
When a prescriber decides to formalize this MBH with an MA the rules as described above apply. Elaborated below, in order of RegistrationDateTime (MA or TA) / MedicationUseDateTime (MGB):
- MGB – MGB – MA
- MGB – MGB – MA - MGB
- MGB – MGB – MA – TA - MGB
- MGB – MGB – MA – MGB - TA - MGB
- MGB – MGB – MA - MGB - MGB
5.3.2.3 Future medication agreements and administration agreements
An MBH may include more than one actual MA and toedieningsafspraak. Namely one for the current situation and one or more for the future situation. This section describes an example with one current and one future. For clarity, the future building blocks have a “T-” in the examples. Both current and future building blocks are relevant for the medication overview. The building blocks for TA and MGB must have adequate references to either the current or future appointments. Below are three examples, in order of RegistrationDateTime (MA or TA/ MedicationUseDateTime (MGB) (if these are equal, then sorted by PeriodOfUse):
- MA – T MA
- MA – TA – T MA – T TA
- MA – TA – MGB – T MA – T TA – T MGB
Suppose that the TA changes because the provider supplies a different strength (2x 500mg tablets instead of 1x 1000mg tablets) and the patient still has enough stock for two weeks based on the first TA:
- MA – TA – MGB – T TA – T MGB
5.3.2.3.1 New medication agreement
If a new MA is created in this situation, a (technical) stop/cancel-MA must always be created as well. This stop/cancel-MA has two appearances:
1) With RelationMedicationagreement to the specific MA that is discontinued or cancelled;
2) Without RelationMedicationagreement. With this the entire MBH is discontinued. This is allowed only when there is no MA available in the MBH.
Re 1) Stop-MA for one current MA. With this stop-MA, a specific MA is discontinued. For that MA a new MA is then created. If only the current MA is changed, this affects the examples given above as follows, in order of RegistrationDateTime:
- MA – T MA - stop-MA – MA
- MA – TA – T MA – T TA - stop-MA – MA
- MA – TA – MGB – T MA – T TA – T MGB - stop-MA – MA
If only the future MA is changed, this results in the following changes applied to the examples above, in order of RegistrationDateTime:
- MA – T MA - cancel-MA – T MA
- MA – TA – T MA – T TA - cancel-MA – T MA
- MA – TA – MGB – T MA – T TA – T MGB - cancel-MA – T MA
Re 2) Stop-MA for the entire drug treatment. This should be created only when there is no MA available in the MBH to refer to with RelationMedicationagreement. This may be the case when the MBH was started with another building block, such as a TA or MGB, or if the MA - for whatever reason - was not delivered upon consultation. In these cases, the system does not "know" this MA and therefore cannot refer to it with a RelationMedicationagreement. A prescriber can then stop the MBH with a stop-MA that has no reference to an MA. If desired, the prescriber can subsequently create a new MA. This is detailed below for the same three examples as above, in order of RegistrationDateTime (MA or TA)/MedicationUseDateTime (MGB).
- TA – stop-MA (without reference) - MA
- TA – T TA – MGB - stop-MA (without reference) - MA - T MA
- TA – MGB - T TA – T MGB - stop-MA (without reference) – MA - T-MA
5.3.2.3.2 New administration agreement
However, you can also make a new TA for the current MA only. This new TA must then have a RelationMedicationagreement to that current MA. Elaborated below for three examples, in order of RegistrationDateTime (MA or TA)/MedicationUseDateTime (MGB).
- MA – T MA – TA
- MA – TA – T MA – T TA – stop-TA – TA
- MA – TA – MGB – T MA – T TA – T MGB – stop-TA – TA
A new TA with a RelationMedicationagreement to the future MA looks like this:
- MA – T MA – TA – T TA
- MA –TA – T MA – T TA – cancel-TA - T TA
- MA – TA – MGB – T MA – T TA – T MGB - cancel-TA – T TA
5.3.2.4 Rules of overruling listed
Based on the above description/examples, the following general rules of overruling can be derived. These are the rules for determining whether a building block is relevant for delivery in the medication overview. Within an MBH:
- A new MA overrules all building blocks of types MA and TA(s), belonging to the MA being changed, stopped or cancelled.
- A new TA overrules the building block of type TA belonging to the TA being changed, stopped or cancelled.
- A new MA or TA always overrules the building blocks of type MGB.
- A new registration of an MGB overrules in principle (see exception below) older registrations of MGB belonging to the same MA as the new MGB.
- Exception to this rule: MGB authored by the patient does NOT overrule an older MGB record authored by a healthcare provider and vice versa. Therefore both are relevant to the medication overview.
The last exception is further applied to the following cases:
- MA – TA – MGB-ZVL – MGB-PAT
- MA – TA – MGB-PAT – MGB-ZVL
- MA – TA – MGB-PAT – MGB-PAT
- MA – TA – MGB-ZVL – MGB-PAT – MGB-PAT
- MA – TA – MGB-ZVL – MGB-PAT – MGB PAT – MGB-ZVL
5.4 Verification per MBH
A health professional records verification per MBH with the MGB building block:
- Author = Health professional
- Possible informant: the patient, a care provider or another person
- Possible characteristic "according to agreement": according to the author, is the medication use in accordance with the MA or TA?
- Possible RelationMedicationagreement or RelationAdministrationagreement:
- MA or TA that has been the reference for recording this MGB.
- If indicated "according to agreement", this is the MA or TA according to which the patient uses.
Such a record of MGB means: "I think the patient uses this drug as follows: ...".
Known: it is not currently provided in the information standard to indicate that an MA or TA is correct, regardless of whether the patient uses it as such.
5.5 Process of the medication overview exchange
In addition to the content and verification, the process surrounding the medication overview is also important.
5.5.1 Who, when, with what information
The reliability and completeness of a medication overview depends on:
- who composed it,
- at what time and
- with what basic information.
We can make agreements about this, for example:
- only exchange/make available a medication overview once it has some form of reliability
- after an explicit action by a health professional or
- automatically if certain conditions are met.
- fully automatically exchange/make available medication overview without conditions
- make medication overview fully automatically available without the need for a new data type (so when registering one of the other medication data types you are automatically also the source of a medication overview).
5.5.1.1 Decision
The above questions have not yet been answered. It has been decided that during the Proof-Of-Concept all parties that can provide a medication overview will do the same.
5.5.2 Responsibilities applicant and source
The applicant receives medication overviews from multiple sources. Processing this is the responsibility of the recipient. The topicality of a medication overview is important here.
Action: the transaction Medication overview must contain the most recent date of the collection of available RegistrationDateTime/MedicationUseDateTime of the delivered building block instantiations. The compiler (source) of the medication overview must therefore provide this date. This helps the recipient to better estimate the topicality of the medication overview.
5.5.3 Agreements continued
We continue the analysis with the results of the Proof-Of-Concept to arrive at a good process for the medication overview.
5.6 Inference rules
It is important that all parties, physician, pharmacist, nurse as well as patient can infer from the medication building blocks what is intended (i.e. what the current agreements are) and that this should be the same for all information systems. For that reason, information systems must be able to calculate the current situation using the same inference rules.
The current therapeutic situation is calculated on the basis of MAs and TAs. TAs have a relationship with the MA that they were added to. For this reason, TAs can be linked to the MA that they belong to.
Of course it is also useful to have information about medication use. However, this does not indicate the aim of the health professional, only what medication the patient has used. For that reason, the rules below do not take MGB in consideration. However, MGB is part of a medication profile but is shown separately under the MBH concerned.
5.6.1 Effective period
The effective period lies between an effective start date and an effective end date.
- The effective period describes the period to which an MA or TA (ultimately) applies.
The effective period depends on adjustments (modifying/discontinuing/halting/resuming) of medication and by the ‘actual handling of an MA by a TA. The effective period is not described in the dataset, because it is inferred which is only used to determine the current situation. The effective start date, effective end date and effective period are not data to be shown to the end user. Medication for an indefinite period has an effective end date that is in the future, but at a time that cannot be distracted (yet). It is only known that it is 'somewhere' in the future.
5.6.2 Actual and current medication
- Actual MAs and TAs: the agreements of which the effective end date lies in the future.
- Current MAs and TAs: the agreements for which the present date lies between their effective start date and the effective end date.
- Actual medication: the collection of actual (current and future) MAs and TAs as well as current MGB. It should be taken into account that it is never possible to say with certainty to what extent the patient complies with the agreements and/or reports actual use of medication.
5.6.3 Details
Approach
Using a number of different situations the inference rules are described for arriving at the effective therapeutic period in a medication profile:
- 1) Starting medication
- 2) Creating an administration agreement
- 3) Changing medication
- 4) Discontinuing medication
- 5) Temporarily halting and resuming medication
The inference rules are described below. The number for each inference rule indicates the order in which the rules must be executed.
1) Starting medication
Medication is started by creating a new MA. The MA has:
- RegistrationDateTime - the date on which the agreement was made with the patient.
- PeriodOfUse - this period consists of:
- startDateTime - the startDateTime of the MA may be in the future;
- Duration - the duration of medication use;
- endDateTime – the date until which the MA is valid.
1a: The effective period of a new MA is equal to its period of medication use.
2) Creating an administration agreement
A TA specifically fulfils an MA. From a patient perspective, the TA, in a way, replaces an MA, as the TA is a more specific indication of what the patient should be using.
Inference rule 1 is extended:
1b: The effective period of a new TA is equal to its period of medication use
Inference rule 2 is applicable when an MA has underlying TAs (see inference rule 2b for further explanation):
2a: The effective period of an MA is equal to the effective period of the TA.
3) Changing medication
- a) By means of an MA
The prescriber changes the medication (i.e. the MBH) by creating a new MA (and discontinuing the existing one) within an existing MBH. Because the startDateTime of an MA may lie in the future, several MAs may be actual at the same time. For example, when an MA applies ‘for an indefinite period’ (MA1) and it is agreed to change the dosage in two weeks (MA2), the first MA (MA1) remains valid for two weeks, after which the second MA (MA2) becomes effective. The previous inference rules do not change because of this.
- b) By means of a TA
A TA specifically fulfils an MA. This TA may be modified. For example, when administration schedules are changed (when GDS is initiated), or when a commercial product is changed (for example, as a result of a preference policy). Several successive TAs can therefore be created under a single MA. Hence, rule 2 is expanded so that the effective period of the MA is determined by the entire series of underlying TAs.
2b: When several TAs are made under a single MA, the effective start date of the MA is then equal to the earliest startDateTime of the underlying TA and the endDateTime of the last TA.
Parallel TAs may exist under a single MA of which the RegistrationDateTime and the startDateTime are the same. Both are then valid at the same time and this does not change rule 2b.
The diagram below includes a simplified example of an actual profile composed of different building blocks. The patient, verification, CiO and lab data have been excluded from this overview. Only limited data has been included, mainly to demonstrate the way in which the effective period works. Lines starting with ‘-’ are detail lines which can be hidden in the medication profile, if required. The first line shows the effective period for the underlying MAs and TAs. The MGB is also displayed, but this does not influence the calculation of the effective therapeutic period on the first line.
4) Discontinuing medication
Discontinuing medication (i.e. discontinuing the MBH) is done by creating a new MA in which stop type ‘discontinued’ is indicated. The startDateTime of this MA is the date on which the original MA started. The endDateTime is the date on which the medication is discontinued. The effective period of the original MA is so replaced by the effective period of this new stop-MA.
1c: When an MA is discontinued, the effective period of the MA is then the effective period of the stop-MA.
A stop-TA fulfils the stop-MA. In this way, for example, it is possible to indicate that the discontinuation starts when the next GDS roll is supplied.
5) Temporarily halting and resuming medication
Temporarily halting medication is carried out in the same way as discontinuing medication. A stop-MA is created (stop type ‘suspended’) with the same startDateTime as that of the original MA. The endDateTime of the stop-MA is the time of the temporary halt, the stop type is ‘suspended’. Resuming medication is done by creating a new MA with the old (or adjusted) dosage with the date of resuming as the startDateTime. A meaningful reason (example: resume previous policy) may serve to clarify matters. If the medication is not resumed, the medication should be stopped with stop type ‘discontinued’, in order to permanently end the MBH, which means the medication is no longer actual. Medication that is halted remains actual, and so remains visible in the medication profile.
1d: When medication is temporarily halted, the effective period is not affected. The effective period is determined by the effective period of the original MA.
These inference rules are the basis for determining the current agreements. It is possible to conceive exceptional situations relating to a partial availability of medication data. These situations have not been elaborated. All inference rules are included and implemented in the open-source medication viewer.
5.7 Data that need not be shown
Various data are important for the correct processing of information, but are not relevant to the end user:
- An application does not have to show the (technical) identification of an MBH (nor that of the building blocks themselves), but the elaboration thereof: namely, building blocks shown in coherence.
- The copy indicator for the MA, TA and MGB when consulting the medication overview. Whether a building block is a copy is not that important to a user and can be deduced by other data from that building block (such as the author of the building block). So it is not so important that a user can see that this has been delivered 'as a copy' and is probably only confusing. However, this information is important for suppliers, for example because they sometimes want to perform calculations with the data and it is then safer to collect the data from the real source. It is therefore obvious to keep track of whether you have received data from the real source in your information system.
- An application does not have to show the stop type (suspended/discontinued) of a stop- or cancel-agreement, but it does have to show how it affects how the MBH is displayed in the medication overview. The effect may be that the entire MBH is shown as stopped medication, halted medication (remains viewable under current medication), a change in case of a 'technical stop-agreement’. In the case of a 'cancel-agreement', the medication will never have been started and therefore will not be shown in a medication overview.
6 Administration list and inference rules
|
This chapter is under revision and will be rewritten taking the contextual dependance of shown information into account |
This chapter shows a possible presentation/view of the administration list and, additionally, specifies the inference rules to identify the relevant medication building blocks within a pharmaceutical treatment, for the generation of an administration list.
6.1 Introduction
This paragraph contains an example of an administration list. The administration list shows the most recent medication data at any time of the day, to facilitate adequate medication administration. To ensure safe circumstances in which correct and up-to-date medication data is presented to the (professional) administrator, the administration software must be able to process the medication building blocks and to correlate the medication building blocks, as described in this information standard.
The term ‘administration list’ is defined as a digital list including all medicaments that are prescribed by a prescriber and that have to be administered to a patient/client. This list is generated based on the following medication building blocks: MA, TA, MVE, MTD and WDS.
Sending and/or making medication data available to (professional) administrators provides a comprehensive overview of all medication to be administered. Importantly, this implies that information regarding discontinuation and change of medication with or without medication dispense is available at any time. Changes in medication and variable dosing regimens, for example as prescribed by the thrombosis service, are immediately available for administrators.
In the figures below, the numbered elements are normative; it is mandatory to show those components. The lay-out of the administration list in an information system may differ depending on the type of users or the type of device; this is dependent on the user requirements.
The administration list in this example is described generally. The chapter entails the general end user requirements for the administration list; this chapter does not comprise details on the technical requirements or implementation.
The existing KNMP specification “User requirements specification Medication overview 2.0”, “Guideline Transfer of Medication data in the chain of care, Revision 2018/2019”, and the new insights from the Medication process program have been incorporated in this chapter.
The basis for the administration list entails:
- Medication building blocks
- MA
- TA
- WDS
- MTD
- All current medication; this comprises all MAs, TAs and WDSs that are available and applicable when the medication is administered. The PeriodOfUse defines whether medication should be considered current medication.
- The InjectionPatchSite is consulted based on the AdministrationDateTime.
- (Professional) administrators can distinguish the types of medication (in GDS package or separately) which should be administered to the patient by the data element DistributionForm (medication building block TA) and the data element AsNeeded/Condition in the dosage (medication building blocks MA, TA or WDS). The different types of medication may be presented according to the following categories:
- GDS medication (including discontinued medication in order to show a notification/warning)
- Non-GDS medication
- Non-GDS medication as needed
- The remaining building blocks VV, MVE and MGB are not relevant for the administration list.
Figure 1: Example administration list.
| Nr | Heading | Dataset | ||
|---|---|---|---|---|
| 1 | Type | MA | TA | WDS |
| 2 | Medicine | AgreedMedicine | MedicineForAdministrationAgreement | AgreedMedicine |
| 3 | Prescriber | MedicationAgreement – Prescriber – HealthProfessional | ||
| 4 | Supplier | AdministrationAgreement – Supplier – HealthcareProvider | ||
| Author | VariableDosingRegimen – Author – HealthProfessional (conditional) | |||
| 5 | Start Date | MedicationAgreement – PeriodOfUse | Administration Agreement – PeriodOfUse | Variable Dosing Regimen – PeriodOfUse |
| 6 | End Date/Duration | MedicationAgreement – PeriodOfUse | AdministrationAgreement – PeriodOfUse | VariableDosingRegimen – PeriodOfUse |
| 7 | Description | InstructionsForUse – Description | ||
| Route Of Administration | InstructionsForUse – RouteOfAdministration | |||
| Additional Instructions | InstructionsForUse – AdditionalInstructions | |||
| Dose | InstructionsForUse – DosingInstructions – Dosage – Dose | |||
| Administration Time | InstructionsForUse – Dosing Instructions – Dosage – AdministeringSchedule – Administration Time | |||
| Administration Speed | InstructionsForUse – Dosing Instructions – Dosage – AdministeringSpeed | |||
| Duration Of Administration | InstructionsForUse – Dosing Instructions – Dosage – DurationOfAdministration | |||
| 8 | Injection-Patch Location | MedicationAdministration – InjectionPatchSite | ||
| 9 | Comment | Comment & AdditionalInformation | ||
Table 1: Normative medication data in the administration list.
6.2 Functional specification
In the figures below the elements with normative data are presented. The other elements are not normative (not yet). The numbered elements in the table are normative; the other elements are optional (until further notice).
6.2.1 Heading and general
6.2.2 Remarks
In the comment data element, for example, (limiting) health literacy competences (competences: literacy, calculation and digital skills) can be recorded, which may affect the medication administration. This part is for internal use and is not exchanged (not yet).
NB. This part is not normative yet. This data is derived from the internal information system of the organisation.
6.2.3 GDS medication
GDS (medication distribution system, in Dutch: 'Geneesmiddel Distributie Systeem') is defined as a package in which medicaments are distributed in units per administration moment, labelled with the name of an individual patient/client (KNMP, 2011).
A GDS allows a patient to self-manage their own medication for longer. Whether medication is placed in GDS is determined by a pharmacist. The data element ‘Distribution form’ in the corresponding TA (and also in the MVE, which, however, is not used to compile the administration list) indicates whether GDS medication is involved.
The healthcare field has expressed the wish for a warning signal to be displayed in the event of changed or discontinued medication, stating that the medicine may be in the GDS packaging and that an action may need to be taken on this. Further elaboration on this is needed.
6.2.4 Non-GDS medication
Non-GDS medication is medication which, for various reasons, cannot be included in an automated medication distribution system. The distribution type 'non-GDS' is specified in the data element DistributionForm in the corresponding TA. Furthermore, for non-GDS medication, the data element Condition in AsNeeded remains empty.
6.2.5 Non-GDS medication – as needed
Non-GDS medication as needed comprises separate medication which should be administered only in specific circumstances. The condition for administration of such medicament can be:
- a measured physiological value (plasma glucose level, body temperature, blood pressure);
- a symptom or other condition (headache, itching).
The distribution type 'non-GDS' is specified in the data element DistributionForm in the corresponding TA. Furthermore, the Condition in AsNeeded is filled with 'As needed'.
6.3 Building block instantiations
This paragraph describes which building block instantiations are related to the administration list. This includes only the ‘own’ building blocks (see paragraph 5.3.1) and only the latest, relevant instantiations (see paragraph 5.3.2).
6.3.1 Therapeutic and logistical building blocks
Compiling an administration list involves therapeutic building blocks. Building blocks can be ‘overruled’ based on inference rules. All inference rules as described in Chapter 5 are applicable. In addition, in this paragraph, the inference rules for WDS are described. The inference rules do not apply to MTD, as medication administration is a procedure at a certain time point; in contrast, the MA, TA and MBG comprise agreements valid for a certain period of time. The logistic building block MVE is not needed for compiling an administration list as TA includes the data element ‘DistributionForm’ in which the type of distribution including ‘GDS medication’ is registered.
6.3.1.1 Administration list and inference rules
An administration list can be compiled, for example in the administrator’s information system, based on separate medication building blocks. The medication building blocks may be retrieved from the own information system or from other sources. The transaction Medication data (consulting/making available) is used for consulting and making available individual medication building blocks. In the transaction group Medication data (sending/receiving), separate medication building blocks can be send to another health professional or patient directly (without prior consultation); for example, in the case of discharge or on request of a fellow health professional (for example, a patient sees a GP or pharmacist outside the region; the GP or pharmacist requests the data by telephone and receives the data digitally). Both for Medication data sending/receiving and Medication data consulting/making available, data obtained from other sources is not allowed to be presented in the administration list.
6.3.2 Last relevant
Only the latest relevant medication building blocks (per MBH) are part of the administration list. This paragraph provides a description of the latest relevant building blocks for an administration list comprising medication on prescription. Paragraph 6.3.2.1 describes the situation in which a WDS is applicable. For WDS, the same inference rules apply as for MA. When preparing an administration list, if a WDS is present, this WDS is leading for the InstructionsForUse. All situations without WDS, as described in paragraph 5.3.2, are applicable as well.
Query to identify the correct, relevant and future medication building blocks:
| Nr | Medication building block | Query Parameter | What should be filled in specifically? |
|---|---|---|---|
| 1 | MA, TA, WDS | PeriodOfUse | All medication that should be administered today and, therefore, is applicable: Day = T, startDate = T: 00:00:00 h, endDate = T: 23:59:59 h |
| 2 | MTD | Administration Period | Depending on medical relevance (for example, historical information on InjectionPatchSite may be relevant back to one week ago). For example, if one week is sufficient (another period may be chosen): Day = T, startDate = T-7 day |
If all medication building blocks are collected, the following medication data, depending on the situation, can be used:
| Medication building block | Situation: only MA* | Situation: MA + TA | Situation: MA + TA + WDS |
|---|---|---|---|
| MA | Leading** for compiling the administration list (number: 2, 5, 6, 7, 9, 10) | Data from the prescriber | Data from the prescriber |
| TA | - | Leading** for compiling the administration list (number 2, 5, 6, 7, 9, 10) | Data from the supplier and medicine |
| WDS | - | - | Leading** for compiling the administration list (number 5, 6, 7, 10) |
* Situation in which the pharmacist has not processed the MA yet. ** ‘Leading’ indicates the data elements which are included in the functional requirements (numbers of the elements are provided between brackets).
6.3.2.1 Medication with prescription with a variable dosing regimen
6.3.2.1.1 Most simple ‘happy flow’
The most simple ‘happy flow’ in a pharmaceutical treatment is stated below.
- MA – WDS – TA
In general, an MBH starts with an MA. A TA specifies the MA. In addition, a WDS identifies whether a variable dosing regimen applies. In both the MA and the TA, the instruction ‘according to schedule thrombosis service’ is recorded. Then, in the WDS, the dosing schedule is specified. The three medication building blocks MA, TA and WDS are relevant and are part of compiling an administration list, as indicated in green below.
- MA – WDS– TA
A TA refers to the MA that is specified by the TA. Also, a WDS refers to the MA that is specified by the WDS. A WDS is always related to an MA.
6.3.2.1.2 New WDS
- MA – WDS – TA – WDS
The PeriodOfUse of the WDS has ended and a new dosing schedule is created. For a variable dosing schedule in which a endDateTime has already been agreed, no additional stop-WDS is required.
6.3.2.1.3 Change of WDS
- MA – WDS– TA – stop-WDS – WDS
The WDS is changed.
6.3.2.1.4 Discontinuation of medication
- MA – WDS – TA – stop-MA
Medication with a WDS is discontinued by stopping the MA.
6.3.2.1.5 Change of trade product
- MA – WDS – TA – stop-TA – TA
The trade product is changed; this does not affect the WDS.
6.3.2.2 Rules of overruling listed
Based on the above descriptions/examples, the following general rules of overruling can be derived. These are the rules for determining whether a building block is relevant for delivery in the administration list. Within an MBH:
- A new MA overrules all therapy building blocks (MA, TA, WDS) belonging to the MA that is being changed, stopped or cancelled.
- A new TA overrules the TA type building block belonging to the TA being changed, stopped or cancelled.
- A new WDS overrules all older WDSs belonging to the same MA.
7 Information systems and transactions
This chapter contains a list of all system roles and transactions that are referred to in the various process chapters.
The figure below (Figure 10) contains an overview of all information systems with their corresponding system roles (for the system roles related to the transfer of laboratory data, we refer to the functional design Information standard exchange Laboratory data). The system roles have been described in Table 3. The system roles associated with consulting/making available medication data and the medication overview are important for all information systems depending on the process in which they are used. The electronic prescribing system (EVS) also includes the system roles for receiving a VVV, sending an AVVV and receiving a VMA and, in an outpatient situation, for sending a prescription and receiving data on the processing of a prescription. In addition to the basic system roles, a XIS and PGO also include the system roles for sending a VMA and a VVV, for sending data on MGB and for receiving an AVVV. The pharmacist information system (AIS) also includes, in addition to the basic system roles, the roles for sending a VMA, sending a VVV and data on processing of the prescription, and receiving a prescription and AVVV. Chapter 2 lists the main system roles per process.
| System role name | Abbr. | Description |
|---|---|---|
| MedicatieGegevensBeschikbaarstellend | MP-MGB | Making medication data available to fellow health professionals/patient |
| MedicatieGegevensRaadplegend | MP-MGR | Consulting medication data at fellow health professionals/patient |
| MedicatieGegevensSturend | MP-MGS | Sending data to fellow health professional/patient |
| MedicatieGegevensOntvangend | MP-MGO | Receiving medication data from fellow health professional/patient |
| MedicatieOverzichtBeschikbaarstellend | MP-MOB | Making medication overview available to fellow health professionals/patient |
| MedicatieOverzichtRaadplegend | MP-MOR | Consulting medication overview at fellow health professionals/patient |
| MedicatieOverzichtSturend | MP-MOS | Sending medication overview to fellow health professional/patientf |
| MedicatieOverzichtOntvangend | MP-MOO | Receiving medication overview from fellow health professional/patient |
| VoorschriftSturend | MP-VOS | Sending dispense request or request for processing modifications to medication agreement |
| VoorschriftOntvangend | MP-VOO | Receiving dispense request or request for processing of modifications to medication agreement |
| VoorschriftAfhandelingSturend | MP-VAS | Sending data on processing of medication agreement and possibly dispense request in administration agreement and/or medication dispense |
| VoorschriftAfhandelingOntvangend | MP-VAO | Receiving data on processing of medication agreeement and possibly dispense request in administration agreement and/or medication dispense |
| VoorstelVerstrekkingsverzoekSturend | MP-VVS | Sending proposal dispense request to prescriber, receiving reply to proposal dispense request |
| VoorstelVerstrekkingsverzoekOntvangend | MP-VVO | Receiving proposal dispense request, sending reply to proposal dispense request |
| VoorstelMedicatieafspraakSturend | MP-VMS | Sending proposal for new or modified medication agreement, receiving reply to proposal medication agreement |
| VoorstelMedicatieafspraakOntvangend | MP-VMO | Receiving proposal for new or modified medication agreement, sending reply to proposal medication agreement |
Table 3 Overview system roles
Table 4 gives an overview of all transaction groups, transactions, corresponding system roles and building blocks exchanged with this transaction group. The names of the transaction groups and transactions link to the ART-DECOR publication which details per scenario which data elements are used (for the transaction groups, transactions and associated system roles related to the transfer of laboratory data, please refer to the functional design Information standard exchange Laboratory data).
Table 4 Overview transaction groups. For PULL transaction groups, sending and receiving are the same transaction
Chapter 2 lists for each process only the relevant transactions per process step. The transaction groups are not included in this. Table 4 includes all transaction groups and transactions.
8 Functionality
This chapter describes indications for the functionality of an information system.
8.1 Filtering medication from 2nd/3rd line (all information systems)
An institution makes all its medication data (all building blocks) available. Not all data may be relevant for the receiving health professionals. Receiving information systems may therefore include a filter depending on the requirements of the specific health professional/patient. The list below (Table 5) includes medication types of which it has been determined that medication administration during admission is relevant for health professionals outside the institution.
| ATC-code | Name |
|---|---|
| L01 | Cytostatics |
| L03AX03 | BCG vaccine, urology |
| L04AA23; L04AA25; L04AA26; L04AA33; L04AA34; L04AB; L04AC | Immunosuppressive agents |
| B03AC; B03XA; B06AC | Iron, erythropoietin, drugs used in angioedema |
| G03GA; G03GB02 | Gonadotropins (HCG, etc.), clomiphene |
| H01CA; H01CC; L02AE04 | Gonadorelin, hypothalamic hormones, triptorelin |
| J06; J07 | Immunoglobulins, vaccines |
| M03AX01 | Botulinum toxin |
| R03DX05 | Omalizumab |
| S01LA04; S01LA05; S01LA | Ophthalmological antineovascularisation agents |
Table 5 Types of clinical medication relevant for external health professionals
8.2 Making medication data available (all information systems)
All own medication data may be made available if the patient has given permission (in accordance with current laws and regulations). Data received from others (healthcare providers/patient) that one has copied into one's own information system and marked as a copy are not made available. The only exception is the transaction medicatieoverzicht (Medication overview) PUSH and PULL, in which case third parties’ building blocks are made available on the condition that they include the original identification label (see also paragraph 5.1).
8.3 Notification date or dispense date (pharmacist information system)
The RequestDate and the actual MedicationDispenseDateTime may be recorded in the MVE. The RequestDate is the time at which a pharmacy records an intended medication dispense. The MedicationDispenseDateTime is the date on which the medication dispense has actually taken place.
When a VV is processed immediately and the patient picks up the medication that same day, both dates will be the same. When the patient picks up the medication one or several days later, different dates must be entered. When medication is dispensed on several occasions as part of the same VV (for example, when prescriptions are split), each MVE has its own RequestDate. When an automated dispense system is being used, the MedicationDispenseDateTime is the time at which the patient picks up the medication from the dispense system. Until this time is available in the pharmacist information system, the time at which the medication is deposited in the automated dispense system may be used. See also paragraph 4.2.5.
8.4 Updating after system malfunction
After a system malfunction, the prescriber’s information system needs to determine whether changes have been made to MAs. For practical examples and rationale, see paragraph 4.1.24 and paragraph 4.1.25.
8.5 Construction for ‘once every 36 hours’ interval
When the dosing instruction reads ‘1 tablet every 36 hours’, this may normally be recorded in one MA (dose: 1 tablet, interval: 36 hours). However, when an information system does not go beyond an interval of, for example, 24 hours, another solution needs to be found.
Variation 1: Making use of an ‘AsNeeded’ instruction with the Condition: ‘36 hours have passed since the last medication administration’. This does however come with a certain amount of freedom that may not be desirable.
Variation 2: A repeating dosing schedule can be created, alternating between administration in the morning, administration in the evening and a day without medication administration:
Repeat period: 3 days Dosage instruction Sequence number: 1 Dosage duration: 1 day Dosage Dose per administration: 1 tablet Administration time: 9 am (per example, this could also be a part of the day) Sequence number: 2 Dosage duration: 1 day Dosage Dose per administration: 1 tablet Administration time: 9 pm (per example, this could also be a part of the day)
If it is not technically possible to choose a 36-hours interval, variation 2 applies.
8.6 EVS / HIS processing Regulation processing
The pharmacist sends all MVEs and changes in TAs to the prescriber via the transaction Afhandelen medicatievoorschrift (settlement prescription). This handling must be automatically processed in the prescribing information system. The prescriber only assesses the TAs and does not have to assess all the MVEs.
8.7 Pages with additional information relevant to the Kickstart
Currently, (2023 - 2024), the information standard MP9 is being tested during the Kickstart. For this phase, additional information has been described that is supportive of the information standard, but partly not yet fully established. These include the following pages:
- Raadplegen en reconciliëren (Consultation and reconciliation)
- Migratie/hybride (Migration/hybrid situation)
- Meesturen nierfunctiewaarde met voorschrift (Send kidney function value with prescription)
- Voorbeelden doseringen - zowel functionele als technische uitwerking in HL7v3 CDA en FHIR (Examples of doses - both functional and technical elaboration in HL7v3 CDA and FHIR)
- Consolidatie/afleidingsregels (Consolidation/deduction rules)
- Workflow and scope for infusion thearpy during the Kickstart
See also the landingspagina voor de Kickstart (landing page for the Kickstart) and Gebruikerseisen en -wensen (User requirements and wishes) for further documentation.
8.8 Medication use: UseIndicator, AsAgreedIndicator, MedicationUseStopType, PeriodOfUse and DosingInstructions
| Situation | 1. Medication is/will be used during PeriodOfUse according to agreement | 2. Medication is/will be used during PeriodOfUse, but not according to agreement | 3. Contrary to agreement, medication is/will not be used during PeriodOfUse | 4. Medication is/will not be used during PeriodOfUse, which is according to agreement | 5. Medication is used during PeriodOfUse but it is unknown if this is according to agreement (self-medication) |
| UseIndicator (is this product being used) |
Yes | Yes | No | N/A (because this would lead to a double negative otherwise) | Yes |
|---|---|---|---|---|---|
| AsAgreedIndicator | No | No | No | Yes | - |
| MedicationUseStopType | N/A | N/A | Discontinued or Suspended | Discontinued or Suspended | N/A |
| StopType depends whether agreement is being discontinued or temporarily halted | StopType in accordance with stop-agreement | ||||
| PeriodOfUse | In accordance with MA or TA | Max. 2 out of 3 | Max. 2 out of 3 | Max. 2 out of 3 | Max. 2 out of 3 |
| startDateTime | The time at which the medication use was started. | The time at which the deviating medication use was/will be started. . | Contrary to agreement, medication is/will not be used during PeriodOfUse | The time at which the medication use was/will be discontinued. | The time at which the medication use was started. |
| Duration | The intended duration of medication use. | The intended duration of deviating medication use. | The (intended) duration of non-use | The (intended) duration of non-use. | The intended duration of medication use. |
| endDateTime | The time at which the period of medication use ends (or has ended or will end). | The time at which the period of deviating medication use ends (or has ended or will end). | The time at which the medication use was/will be resumed (or is intended to resume). | The time at which the medication use was/will be resumed (or is intended to resume). | The time at which the period of medication use ends (or has ended or will end). |
| DosingInstructions | In accordance with MA or TA | Required | N/A | N/A | Required |
| Medication was/will be used during the PeriodOfUse according to the agreement, therefore dosage is known. | Medication was/will not be used according to the agreement, the actual dosage used must be communicated. | The medication was/will not be used, there is no dosage. | The medication was/will not be used, there is no dosage. | Dosage that the patient has agreed upon himself. |
Examples:
Situation 1a: Medication is/will be used during the PeriodOfUse according to agreement
Patient has been prescribed ferrous fumarate for anaemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a startDateTime of June 4, yyyy and no endDateTime.
On 10 June, the patient registers the MGB for the past period (from 4 to 10 June yyyy).
| Situation 1a | |
|---|---|
| ProductUsed | FERROUS FUMARATE 100MG TABLET |
| UseIndicator | Yes |
| AsAgreedIndicator | Yes |
| MedicationUseStopType | Not applicable |
| startDateTime | 4 June yyyy |
| endDateTime | 10 June yyyy |
| Dosing instruction | According to agreement |
Situation 1b: Medication is/will be used during the PeriodOfUse according to agreement
Patient has been prescribed ferrous fumarate for anaemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a startDateTime of June 4, yyyy and no endDateTime.
On 10 June, the patient registers the MGB for the past period (from 4 to 10 June).
| Situation 1b | |
|---|---|
| ProductUsed | FERROUS FUMARATE 100MG TABLET |
| UseIndicator | Yes |
| AsAgreedIndicator | Yes |
| MedicationUseStopType | Not applicable |
| startDateTime | 4 June yyyy |
| endDateTime | - |
| Dosing instruction | According to agreement |
Situation 2: Medication is/will be used during the PeriodOfUse, but not by agreement
Patient has been prescribed ferrous fumarate for anaemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a startDateTime of June 4, yyyy and no endDateTime.
The patient is on the road a lot during the day and forgets for a week to take the medicines for lunch. However, the tablets are taken in the morning and evening before eating.
| Situation 2 | |
|---|---|
| ProductUsed | FERROUS FUMARATE 100MG TABLET |
| UseIndicator | Yes |
| AsAgreedIndicator | No |
| MedicationUseStopType | Not applicable |
| startDateTime | 11 June |
| endDateTime | 15 June |
| Dosing instruction | 1 tablet 2 times a day |
Situation 3: Contrary to the agreement, the medication is not/will not be used during the PeriodOfUse
Patient has been prescribed ferrous fumarate for anaemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a startDateTime of June 4, yyyy and no endDateTime.
The patient goes on vacation for a long weekend and finds out too late that the medication has not been packed. The patient will not take the tablets for the next few days and wants to record it.
| Situation 3 | |
|---|---|
| ProductUsed | FERROUS FUMARATE 100MG TABLET |
| UseIndicator | No |
| AsAgreedIndicator | No |
| MedicationUseStopType | Suspended |
| startDateTime | 20 July |
| endDateTime | 23 July |
| Dosing instruction | Not applicable |
Situation 4: Medication is not/will not be used during the PeriodOfUse and that is also by agreement
Patient has been prescribed ferrous fumarate for anaemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a startDateTime of June 4, yyyy .
On August 12, it turns out after checking that there is no longer anaemia and the doctor stops the medication. The patient wants to register that she has stopped taking the medication and registers this herself on August 15.
| Situation 4 | |
|---|---|
| ProductUsed | FERROUS FUMARATE 100MG TABLET |
| UseIndicator | No |
| AsAgreedIndicator | Yes |
| MedicationUseStopType | Discontinued |
| startDateTime | 12 August yyyy |
| endDateTime | - |
| Dosing instruction | Not applicable |
Situation 5: Medication is/will be used during the PeriodOfUse, but it is unknown whether this is by agreement or that there is no agreement (self-medication)
Patient has the flu and he takes paracetamol 500 mg from the local drug store for fever and pain. He has been taking 4 to 6 tablets a day for four days and expects to do this for another three days. He registers this on August 12 as MGB.
| Situation 5 | |
|---|---|
| ProductUsed | Paracetamol 500mg |
| UseIndicator | Yes |
| AsAgreedIndicator | - |
| MedicationUseStopType | Not applicable |
| startDateTime | 9 August yyyy |
| Duration | 7 days |
| Dosing instruction | 4 to 6 tablets a day |
8.9 Implementation of medication distribution system (GDS) data elements
8.9.1 Background
More and more patients are receiving their medication through medication distribution systems (in Dutch “Geneesmiddel Distributie Systeem"), with the pharmacist providing patients with an overview and organisation of their drugs by preparing these in separate compartment units. This form of delivery took off when the Medicines Act in 2007 stipulated that in healthcare institutions without a pharmacy, the medication should be individually registered for the patients.
It is important for health professionals to know whether a patient is using GDS. In 2018, an analysis was conducted (under the direction of Z-Index, with health professionals) of the bottlenecks and wishes regarding the recording of a patient using GDS. Overall, it has emerged that it is desirable to be able to see whether a patient is using GDS at both the medication level and the patient level. For details see next paragraph.
The directions below provide guidance on how the Medication Process can support these needs.
8.9.2 Wishes of care providers
In 2018, an inventory was made with the user councils of Z-Index about the wishes regarding the recording and exchange of GDS. Below is an overview of these wishes.
Who
- All healthcare parties (from prescriber to operator)
What
- Determines which pharmacy delivers
- Pharmacy must know whether the change applies to the current roll or not
- Important for stopping previous medication at the start of GDS
- Evaluation of GDS patients for health insurer
- Other logistics processes towards home care / informal care
Why
- Determines which pharmacy delivers
- Pharmacy must know whether the change applies to the current roll or not
- Important for stopping previous medication at the start of GDS
- Evaluation of GDS patients for health insurer
- Other logistics processes towards home care / informal care
When
- When starting medication, in a pharmacy already before notification of prescription
- When transferring 1st / 2nd line
- When changing (incl. Stopping) medication
- When changing CiO data
Where
- When working in the patient's file, it should be clear that this characteristic applies to the patient concerned. This can be different for each type of care provider (patient file, medication file). The wish of public pharmacists is that they always have this characteristic available for a specific patient.
- On medication overview
8.9.3 Data elements Medication process
The building blocks for the Medication Process have the following data elements that play a role in recording GDS.
| Building block | Data element nr | Description | Type of content |
|---|---|---|---|
| MA | 23283 | AdditionalInformation | AdditionalInformation contains a list of details of the medicatieafspraak that are important for pharmacovigilance monitoring and completion by a pharmacist.
For example: "change in GDS immediately" |
| VV | 22759 | AdditionalWishes | Logistical instructions important for the completion of the dispense request by the pharmacist.
For example: "do not include in GDS" |
| MVE | 20927 | DistributionForm | Distribution form
For example: GDS |
| 20924 | DurationOfUse | Stock for this duration, consumption period | |
| 22500 | RequestDate | The request date is the time when a pharmacist records an intended dispense | |
| 20272 | MedicationDispenseDateTime | The time of dispense. The date/time when the medicine is supplied to the patient |
8.9.4 Application of data elements to support desired functionality for GDS patients
8.9.4.1 Recording that someone uses GDS
When working in the patient's file it is important to see that someone is using GDS. This applies to the pharmacy that supplies the GDS, as well as other care providers of the patient. It is therefore important that this information can be communicated with and shown to other health professionals.
As long as there is no patient characteristic with which GDS can be registered, it can be decided to derive this information from the GDS data that have been recorded with the medication. A possible handle for this is the fact that medication is included in the distribution module together with the DistributionForm data element in the MVE.
- Step 1: if medication is included in the distribution module: fill in the DistributionForm data element in the MVE with 'GDS'.
- Step 2: Based on the DistributionForm data element, deduce that a patient is using GDS. The fact that a patient has an MVE where the DistributionForm data element is filled with "GDS" indicates that the patient is using GDS. This MVE can have been recorded by oneself, or it can have been received from another pharmacy (insofar as this data is collected and recorded in such a way that the contents of the DistributionForm data element are reusable). The fact that a patient uses GDS can then be shown when working in the medication file or the patient file. In consultation with end users, it should be further determined where and how this is shown.
8.9.4.2 Recording why someone uses GDS
Users have indicated that it is desirable to be able to record why someone uses GDS. There are currently no structural data elements available for this.
8.9.4.3 Record for medication that this must be in the GDS
Prescribers want to be able to indicate whether or not a medicine should be supplied via GDS. This can be done as follows:
- VV, AdditionalWishes (AanvullendeWensen) data element. The code list for this data element contains the item "not in GDS".
8.9.4.4 Establishing / exchanging the duration of a medication roll
The duration of the medication roll can be determined on the basis of the "DurationOfUse" of the MVE: the "DurationOfUse" indicates the duration of a medication roll.
8.9.4.5 Establishing / exchanging whether a change in the medication should be effective immediately or not
It is desirable that a prescriber can indicate whether changes in the medication should be implemented immediately or that they can wait until the next roll change. The handles for this are as follows:
- MA, data element AdditionalInformation (AanvullendeInformatie) data element. The code list for this data element contains two items that relate to changes in the GDS, namely "Change in GDS immediately" and "Change in GDS per roll change".
- It is important that the prescriber has insight into the duration of the roll and how long it will take before the current medication roll is used up. This can be deduced from:
- MVE, data element DurationOfUse (Verbruiksduur), see above. For this, the prescribing system must have access to the supply data of previous MVEs.
- MVE, data element MedicationDispenseDateTime (or if this data element is not filled but the RequestDate is, then the RequestDate; if both are filled, MedicationDispenseDateTime is preferred). Based on this date and the DurationOfUse, it is possible to calculate the date on which the current medication roll is used.
9 Reflections
9.1 Prescription without specified recipient (ambulatory)
In this paragraph, the term ‘prescription’ is used to indicate the message through which a patient may pick up a medicinal product from a supplier. A prescription contains an MA and a VV.
In the Netherlands it is customary to digitally send prescriptions to a receiving pharmacy instead of having to collect prescriptions. This requires that the pharmacy is already known.
This paragraph considers whether more flexibility can be achieved in the logistical processing of prescriptions, without cancelling out current advantages. This paragraph may be seen as a long-term vision that we aim towards.
9.1.1 Basic principles
When considering how to achieve flexibility in the processing of prescriptions, these basic principles have been formulated:
- The patient is free to choose where prescriptions are handled
- The current option for sending prescriptions remains possible.
- The information system must reuse the same functionality as much as possible.
- A prescription may only be dispensed once.
Since the methodology described below uses the existing method of sending prescriptions, the same legal rules and regulations apply to prescription validity. All discussions about electronic signatures are equally applicable to the current method of communicating prescriptions.
9.1.2 Details
A patient may choose from 3 options:
- A prescription is always sent to the same regular pharmacist.
- The patient indicates each time where the prescription has to be sent.
- The patient does not give any indication, and goes to a pharmacy which subsequently requests and processes the prescription.
Options 1 and 2 require a patient portal, in which the patient indicates his preference. For option 2, a patient may define a preferred pharmacy which would be at the top of the selection screen when the patient is asked where the prescription must be sent. If the patient does not give any indication, option 3 can be the only procedure.
The method partly uses a concept called “publish and subscribe”.
It is important that the pharmacist explains this principle to his customers.
9.1.3 Work process
A physician decides to create a prescription for a patient. This may be during a consultation or even when repeat prescriptions are requested. The physician registers the prescription in his prescribing system (EPS). The physician does not need to consider the pharmacy as the patient handles this through the portal.
After approval of the prescription, a central prescription index is updated. The EVS automatically sends a notification to this prescription registry to facilitate this. Here, the prescription index is seen as a separate registry with the data type “prescriptions”. It is always possible to consider whether or not this can be merged at a later time. Initially, atomic registration in the registry is being considered. However, at a later date, consideration could be given as to whether categorical registration would be sufficient.
It is important to realise that currently only a notification is given when a prescription is ready. After all, the prescription is still in the EVS database with the status “is available”. There is no prescription message yet.
The prescription registry knows from the patient’s preference settings what needs to be done with the notification:
- With the option ‘send to regular pharmacy’, a notification is sent to the subscription holder of the patient with the message that a prescription can be retrieved.
- With the option ‘patient indicates pharmacy’, the prescription registry sends a notification (e.g. SMS) to the patient. The prescription registry then waits until the patient uses a smartphone app or the patient portal to indicate to which pharmacy the notification should be sent.
- With the option ‘no indication’, the prescription registry does nothing. The patient does not have a regular subscription holder.
9.1.4 Processing by pharmacy
A receiving pharmacy can deduce from the transmitted notification signal (data type) that it refers to an open prescription. The signal also identifies who the patient is as well as the source of the prescription.
For patients who selected option 3 (no indication), the process starts now. The patient identifies himself and notifies the pharmacy that a prescription is ready at a certain institution. The pharmacist may look in the prescription registry to find out which EVS is involved.
The pharmacy information system asks the EVS source system to send the prescription of the respective patient. Since this is a request without medical content, it may even be submitted at lower trust levels.
9.1.5 Sending via EVS
If an EVS receives a request for “sending outstanding prescriptions”, the EVS will check whether prescriptions are indeed outstanding. This prevents a prescription from being retrieved several times. The address of the pharmacy is entered and the prepared prescription message is finally created. The prescription message is sent to the respective pharmacy.
If the prescription has been retrieved before, an error message is sent to the retrieving pharmacy announcing that the prescription is no longer available.
Sending prescriptions is the standard process and already customary for pushing prescriptions. The advantage of this is that the receiving pharmacy only needs to maintain one method for processing received prescriptions. It is noted again that the same legal rules and regulations continue to apply.
After sending a prescription, the EVS also sends a notification to the prescription registry to remove the entry of the outstanding prescription. This indicates that the respective prescription can no longer be retrieved.
10 Appendix References
10.1 General references
| Author(s) | Title | Version | Date (consultation) | Source | Organisation |
|---|---|---|---|---|---|
| NHG, KNMP, Z-index | Building blocks of the medication process | 2014 | Augustus 2015 | https://www.nhg.org/bouwstenen | NHG, KNMP, Z-index |
| ActiZ, KNMP, NVZA a.o | Safe principles in the medication chain | 2014 | August 2015 | http://www.knmp.nl/patiëntenzorg/samenwerking/brochure-veilige-principes-in-de-medicatieketen | ActiZ, KNMP, NVZA a.o. |
| Miscellaneous | Guideline on exchange of medication data in the medication chain | 25 April 2008 | August 2015 | Website KNMP | Miscellaneous |
| Paul Geels | Assessment of medication self-management (BEM) in care homes, Institute for Responsible Medication Use | February 2009 | http://www.instellingsapotheek.nl/downloads/rapporten/beoordeling_eigen_beheer_van_medicatie_in_verzorgingshuizen.pdf | Institute for Responsible Medication Use | |
| ActiZ | Safety in the medication chain | March 2012 | https://www.knmp.nl/downloads/brochure-veiligheid-in-de-medicatieketen.pdf | ActiZ, Miscellaneous | |
| KNMG | Guideline on electronic prescription | September 2013 | Website KNMG | KNMG |
10.2 Qualification scripts
11 Attachment: Document history
| Version | Date | Description |
|---|---|---|
| 0.95 | 15 July 2016 | Pilot version |
| 0.96 | 1 December 2016 | Paragraph 2.4 Process: Administer was adapted and C6: Sending/receiving administration data was added as a result. |
| 0.97 | 22 December 2016 | Paragraph 2.4, 4.3.1 review remarks incorporated. Paragraph 4.2.11 text made more precise. |
| 9.0.2 | 18 June 2017 | Conversion of the document to wiki. |
| 9.0.4 | September 2017 |
|
| 9.0.5 | January 2018 |
|
| 9.0.6 | May 2018 |
|
| 9.0.7 | December 2018 |
|
| 9.0.7 | July 2019 |
|
| 9.1.0 | 29 January 2020 |
|
| 9.1.0 | September 2020 |
|
| 9 2.0.0 bèta | 01 October 2021 | all changes see: *Release notes |
| 9 2.0.0 | 05 April 2022 | all changes see: *Release notes |
| 9 2.0.0 | 08 April 2022 | broken internal links FO corrected BITS MP-607 |
| 9 2.0.0 | 14 April 2022 | Changed images of usecase 4.1.38, 4.1.39 and 4.1.40, they showed GDS instead of WDS BITS MP-534 |
| 9 3.0.0-beta.1 | February 2023 | all changes see: *Release notes |
| 9 3.0.0-beta.2 | October 2023 | all changes see: *Release notes |
| 9 3.0.0-beta.3 | March 2024 | all changes see: *Release notes |
| 9 3.0.0-beta.4 | November 2024 | all changes see: *Releasenotes |
12 Appendix: Figures and tables
Figure 1 Building blocks - overview
Figure 2 Building blocks - coherence
Figure 3 Activity diagram - Medication process in general
Figure 4 Process steps and transactions - medication verification
Figure 5 Process steps and transactions - prescribing
Figure 6 Process steps and transactions - make available
Figure 7 Process steps and transactions - administer
Figure 8 Process steps and transactions - use
Figure 9 Example effective period
Figure 10 Overview of systems and system roles
Figure 11 Interaction diagram Prescribing without address
Table 1 Building blocks - description
Table 2 Informing versus Send and/or make available
Table 3 Overview of system roles
Table 4 Overview of transaction groups
Table 5 Types of clinical medication relevant for outpatient care providers
- ↑ This document only uses the term medication agreement, which therefore also indicates the clinical equivalent provisional medication order
- ↑ The dispense request building block is not applicable in the clinical setting. Dispensing medication is handled in different ways in the clinical setting. Replenishment of, for example, a department’s supply is not considered a medication dispense, but rather an extension of the pharmacist’s stock. Medication dispense only takes place when the link between the medication and patient has been made. In clinical situations, administration often takes place immediately afterwards.
- ↑ 3,0 3,1 A provisional medication order, as it is used in hospitals, is both the request from the physician to the administrator to administer medication to the patient and a dispense request to the pharmacist to ensure that the medication is available for the administrator. This last part corresponds to the medication agreement and the dispense request from the first line. In addition, the hospital pharmacist usually carries out a validation of the administration request (this creates the final medication order which is called an administration agreement here). The provisional medication order is therefore not the same as a proposal from, for example, a nurse on the basis of a protocol that has not yet been approved by a physician.
- ↑ Medication use may have been preceded by medication administration. Registration of medication use, for example after administration of rabies vaccinations or an infusion is not obvious.
- ↑ In practice several MAs (with different PRKs) are often combined into a single product instead of a single MA with underlying the composition.
- ↑ The patient may also verify his own medication. He then records medication use, see paragraph 2.5.4.1.
- ↑ This is about patients from a nursing home or a mental health institution who are going to the outpatient clinic of a hospital.
- ↑ This includes all health professionals who are authorised to prescribe: not only physicians, but also nurse practitioners and physician assistants, for example
- ↑ In the case of substitution, the existing MA is discontinued and a new MA is created under a new MBH.
- ↑ Information systems keep an audit trail. In case of a change, the existing MA is discontinued (new record) and a new MA with the change is then created (new record). By using the records of the audit trail, no major adaptations are needed for discontinuing a MA in most information systems.
- ↑ This does not mean that end users actually have to create two agreements. A user friendly presentation by the information system is desired.
- ↑ Correcting administration agreements happens in the same way. XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ The patient may also request a repeat directly from the prescriber: see paragraph 2.5.6.
- ↑ In this document, the administration list means both the digital and the paper version, unless otherwise indicated. Sublist and checklist are synonyms.
- ↑ In a clinical situation, product may be taken from the department’s own supply, after which administration takes place immediately and the administration is recorded.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ See also paragraph 4.1.3.